[ad_1]
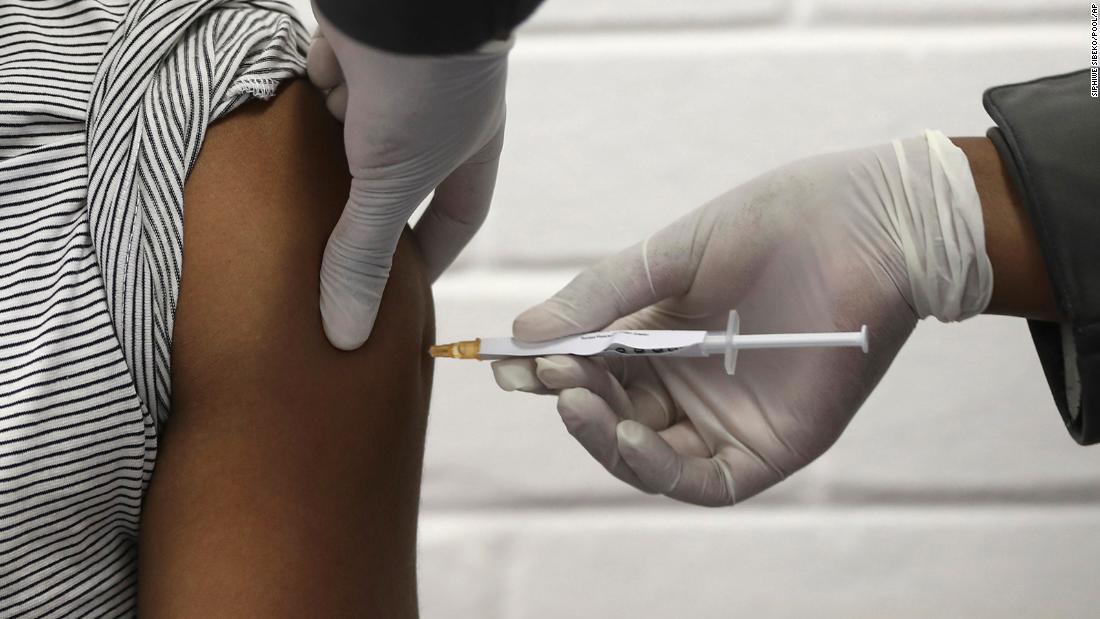
(CNN) – The United States could begin distributing doses of a COVID-19 vaccine “shortly after” December 10, Department of Health and Human Services Secretary Alex Azar said Tuesday.
The U.S. Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee will meet on that date in December to discuss Pfizer and BioNTech’s request for an emergency use authorization for their coronavirus vaccine.
MIRA: This will be the vaccination strategy against covid-19 in Spain
The vaccine could start rolling out in December
“Hopefully, we could distribute the vaccine shortly after December 10,” Azar said during a briefing on Operation Warp Speed on Tuesday.
“We believe we can distribute the vaccine to all 64 jurisdictions within 24 hours of FDA clearance. So we hope the administration can start as soon as the product arrives, “Azar said.” One of the private sector partners we contracted, CVS Health, said they hope to vaccinate nursing home residents, one of the groups with the highest priority, within 48 hours of FDA clearance. “

Source link