[ad_1]
China’s Sinovac Biotech Ltd., which has developed a vaccine against the corona virus, which infects more than 51 million people worldwide and causes the deaths of over 1.2 million people. made a statement …
Yesterday, Pfizer based in the United States and developed by biontech in Germany, the Covidien-19 vaccine 3. success rate of up to 90 percent in the phase conclusions reached explaining, Sinovac’s trial in Brazil of the attempted Coronavac is the vaccine in Turkey discontinued.
Studies on the vaccine developed by China in Brazil have been suspended.
While it was announced that the vaccine was in the last stage, it was claimed that a very serious side effect was seen. Therefore, for the first time, a vaccine developed by China was discontinued due to its side effects.
While the Brazilian Health Agency announced that the trials were halted on October 29, no further details were provided on the matter. The authorities announced that the work continues in parallel with the regulation and that studies will continue following the analysis.
The Bhutantan Institute of Sao Paulo, which produces the vaccine locally, in collaboration with the Chinese company called Sinovac, said: “This decision surprised. We are examining the details of the research ”. The director of the institute, Dimas Covas, announced on a television program that he had witnessed the death of a volunteer who participated in vaccine trials. Covas said this person did not die from the vaccine.
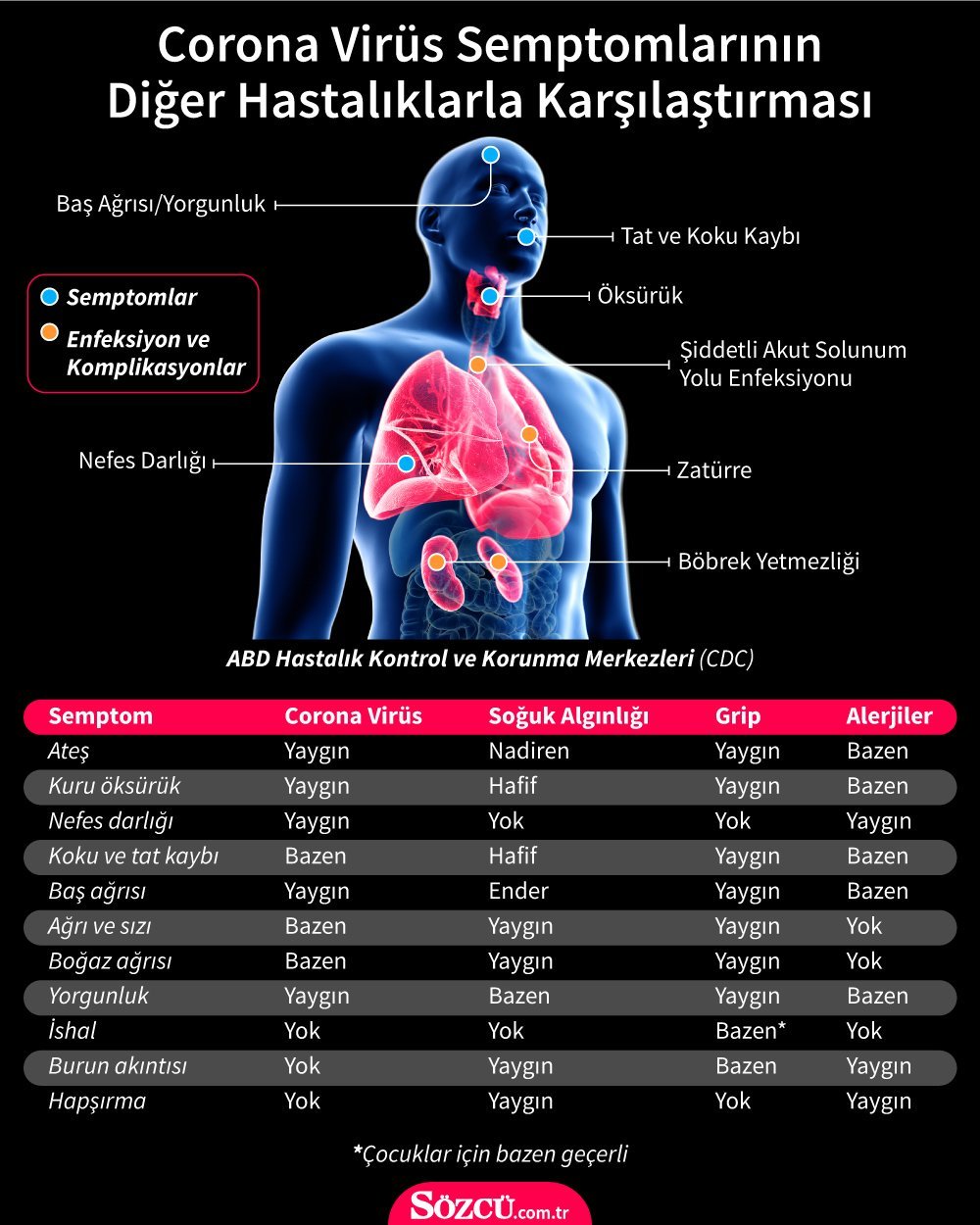
IT WAS BEFORE
Sinovac officials have not made a clear statement on the matter. Previously, the vaccine trials of AstraZeneca and Johnson & Johnson were temporarily suspended, but continued after the investigation. However, the vaccine was registered in China and hundreds of thousands of people have tried this vaccine. Last month, China’s Science Ministry announced that around 60,000 volunteers had tried the vaccine in the last phase of the vaccine and that there were no side effects.
China’s announcement came after Pfizer and BioNTech announced their results in phase three yesterday and announced that the vaccine they developed prevented symptomatic infections by 90%.
13 THOUSAND PEOPLE CALLED IN TURKEY
Coronavac is vaccination, in September the summoning of Turkey also begins … Turkey in the first phase, against Covidien-19 in the highest risk group in 1200, the vaccine in Turkey will be retried in health workers to keep on 13 thousand people.
The study participants are divided into two groups, some are given a real vaccine, while others are given a placebo, that is, an empty vaccine. Vaccinations are observed to affect volunteers. Although volunteers are selected between 18 and 60, people with Covid-19 are not included in the study. Coronavac vaccine, a virus that has lost its infectious properties, is injected into the body and the person is expected to be immune to the disease.
The vaccine is being tested at Hacettepe University and Kocaeli University.
.
[ad_2]
Source link