[ad_1]
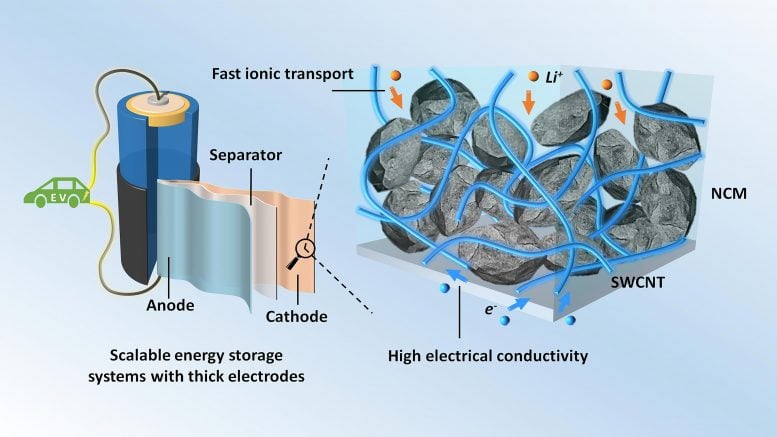
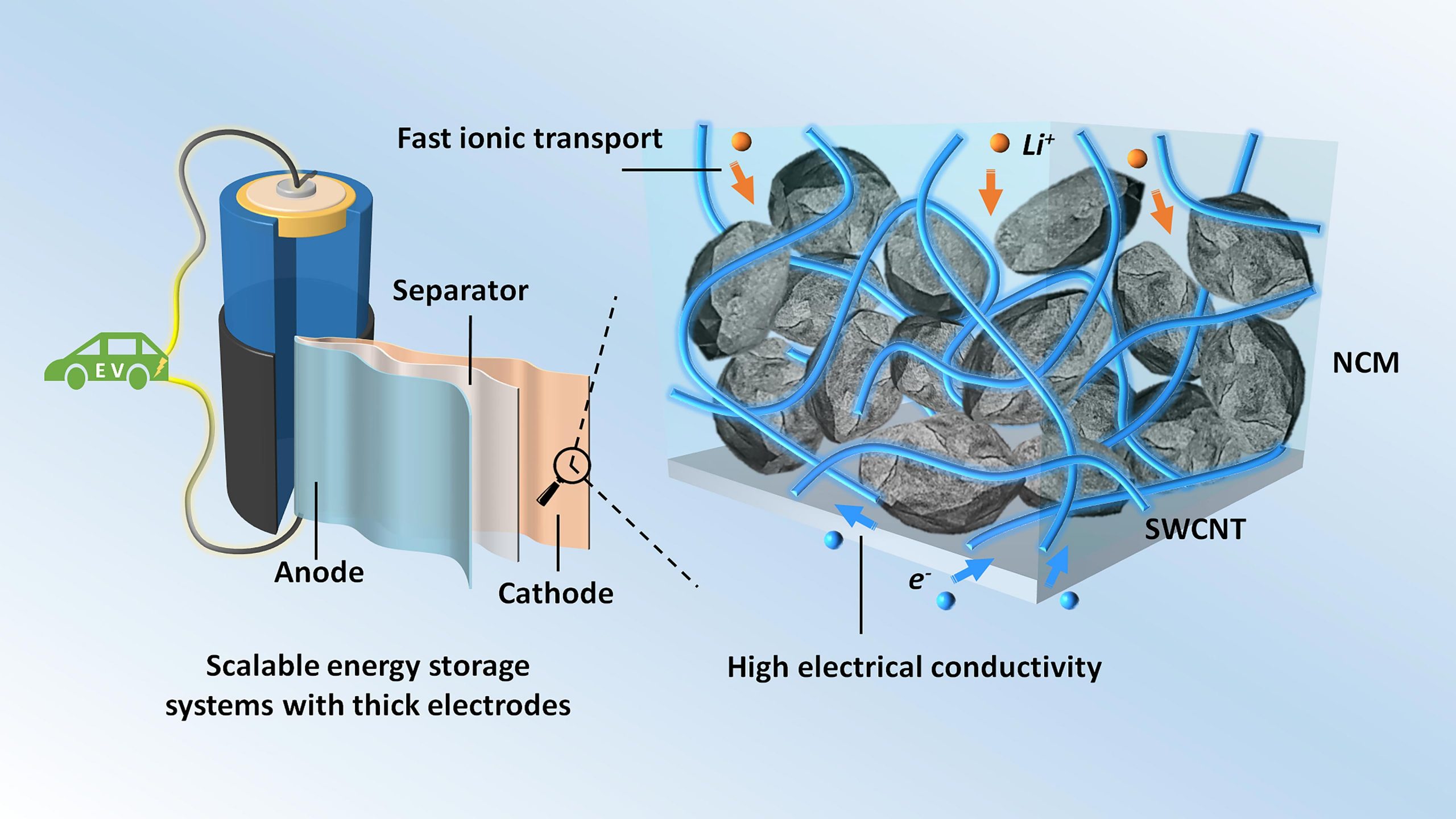
Thick electrodes with single-walled carbon nanotubes (SWCNT) for scalable energy storage systems. Credit: Zhengyu Ju and Guihua Yu
The conductive carbon charges in lithium-ion batteries enable high power with reversible energy storage.
Lithium-ion batteries are the main rechargeable energy source for many portable devices and electric vehicles, but their use is limited because they do not provide high power and at the same time allow for reversible energy storage. Search reported in Applied Physics Reviews, from AIP Publishing, aims to offer a solution by showing how the inclusion of conductive fillers improves battery performance.
The optimal battery design involves thick electrode structures. This increases the energy density, but the design suffers from poor lithium ion transport, a key step in the operation of these electrodes. Various improvement techniques have been tried, including building vertically aligned channels or creating pores of adequate size to facilitate the transport of lithium ions.
Another approach involves the use of carbon charges that conduct electricity. This study considered three types of fillers: single-walled carbon nanotubes (SWCNT), graphene nanosheets and a substance known as Super P, a type of carbon black particles produced during the oxidation of petroleum precursors. Super P is the most commonly used conductive filler in lithium-ion batteries.
The fillers were added to a type of electrode material known as NCM which contains nickel, cobalt and manganese. The researchers examined the resulting composites with scanning electron microscopy. It turned out that the Super P and NCM particles were arranged in a point-to-point contact mode.
The SWCNTs were, however, wrapped around the NCM particles, forming a conductive coating. In addition, interconnected networks of SWCNTs were observed in the spaces between the NCM particles. The graphene nanosheets were also wrapped around the particles of the NCM electrode, but not as uniformly as the SWCNTs were.
SWCNTs were found to be the best conductive filler for NCM electrodes.
“The measured conductivity is consistent with the percolation theory … When an electrically conductive filler is added to an insulating matrix, significant increases in conductivity will occur once the first conductive path through the composite is formed,” said Guihua Yu, a of the authors.
Since percolation requires a full path through the filler, a sufficient amount of conductive filler is required. Therefore, the researchers considered various amounts of filler and found that combining NCM electrodes with just 0.16 wt% SWCNT produced good electrical conductivity. To achieve these same results, larger quantities of Super P and graphene were required.
The researchers used several spectroscopic techniques, including Raman and X-ray absorption spectroscopy, to study the resulting composites.
“This is a collaborative effort of the Center for Mesoscale Transport Properties, an energy frontier research center supported by the US Department of Energy’s basic energy science program. Our results suggest that integration of SWCNT into the NCM electrode facilitates charge and ion transfer. This will lead to increased electrochemical utilization, especially at high discharge rates, “Yu said.
Reference: “Unveiling the Dimensionality Effect of Conductive Fillers in Thick Battery Electrodes for High Energy Storage Systems Presented” by Zhengyu Ju, Xiao Zhang, Steven T. King, Calvin D. Quilty, Yue Zhu, Kenneth J. Takeuchi, Esther S. Takeuchi, David C. Bock, Lei Wang, Amy C. Marschilok and Guihua Yu, November 10, 2020, Applied Physics Reviews.
DOI: 10.1063 / 5.0024123
[ad_2]
Source link