
[ad_1]
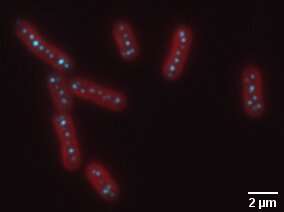
In the photo is a fluorescence image of cyanobacteria, in red, with carboxisomes, in cyan. Credit: Biteen and Vecchiarelli Labs
Organelles within cells are small motors that encapsulate the processes that allow cells to live.
But scientists recently discovered that some organelles are not bound by a membrane, and studying these compartments in bacteria could open the door to understanding how some bacteria thrive and how to counteract others.
Over the past decade, scientists have realized that eukaryotic cells – cells that contain a nucleus and membrane-bound organelles – also use what are called membraneless organelles. These membraneless organelles limit a variety of processes for cells to be able to function properly, says Anthony Vecchiarelli, assistant professor of molecular, cellular, and developmental biology at the University of Michigan.
Now, a review of Unified Messaging conducted by graduate student Christopher Azaldegui and including Vecchiarelli and Julie Biteen, associate professor of chemistry and biophysics, demonstrates how membraneless organelles also operate inside bacterial cells. The review features 10 examples of membraneless organelles found in a variety of bacteria, which can be regulated / formed by a process called liquid-liquid phase separation.
“You can think of it as when you mix oil with vinegar: they both remain liquid, but they separate from each other,” said Vecchiarelli.
Liquid droplets form when biomolecules such as proteins and nucleic acids such as RNA separate from the cell’s cytoplasm. These liquid droplets assemble themselves through weak interactions: protein-protein interactions or protein-nucleic acid interactions. These membraneless organelles are involved in a wide variety of processes in bacteria such as metabolism, chromosome organization, chromosome segregation, cell division, pathogenesis, and DNA replication, translation and transcription.
It is important to understand how these membraneless organelles work because they are much more reactive than membrane-bound organelles to changes in their environment, including temperature, the acidity of the cell cytoplasm, or the availability of nutrients in the cell. For example, Azaldegui describes a transporter in tuberculosis bacterium that can undergo phase separation to assemble the machinery necessary for tuberculosis virulence. Stopping the separation of the liquid-liquid phase will stop the development of the bacterium disease.
Vecchiarelli’s lab in particular studies the carboxisome, a carbon-fixing organelle found in cyanobacteria (often called blue-green algae), a type of bacterium that can cause disease in humans or other animals that encounter it. But the carboxisome converts carbon dioxide from the atmosphere into sugar which cyanobacteria use to grow. Cyanobacteria that feed on atmospheric carbon dioxide play a key role in global carbon sequestration.
“Aside from their ability to produce toxins, cyanobacteria are also responsible for fixing nearly 35 percent of all global carbon, largely due to the carbon-concentrating ability of the carboxysome,” Vecchiarelli said. “Understanding how the carboxisome removes carbon dioxide from our atmosphere certainly plays an important role in understanding how to mitigate climate change.”
Scientists are just starting to identify membraneless organelles in bacteria because the bacteria are much smaller than eukaryotic cells, on the order of 10 to 100 times smaller, Azaldegui says. With this review, Azaldegui hopes to provide a platform for studying membraneless organelles in bacteria in a more standardized way, in this case, using a technique called super-resolution microscopy, a technique he develops in Julie Biteen’s lab.
“By using fluorescence microscopy to precisely detect and pinpoint the position of one molecule at a time, we can resolve the organization and movement, even within bacterial cells. This approach is particularly important because it is compatible with living cells.” said Biteen, an associate professor of chemistry and biophysics.
Lasers and sample preparation do not damage cells, and fluorescence imaging is done in a standard benchtop microscope, as opposed to electron microscopy which requires a vacuum atmosphere in which cells cannot live.
“In Professor Biteen’s lab, we have developed super-resolution microscopy tools that break the conventional limit of resolution to actually see structures at a 10- to 30-nanometer scale,” Azaldegui said. “I started thinking about how these tools would be very useful in studying membraneless organelles and how I can develop a more rigorous and quantitative way to evaluate these droplets in bacteria.”
The endoplasmic reticulum has been found to come into contact with at least two membrane-free compartments and influence their behavior
Christopher A. Azaldegui et al. The emergence of phase separation as an organizing principle in bacteria, Biophysical Journal (2020). DOI: 10.1016 / j.bpj.2020.09.023
Provided by the University of Michigan
Quote: Understanding the ‘membrane’ in membraneless organelles (2020, November 13) retrieved November 13, 2020 from https://phys.org/news/2020-11-membrane-membraneless-organelles.html
This document is subject to copyright. Apart from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link