
[ad_1]
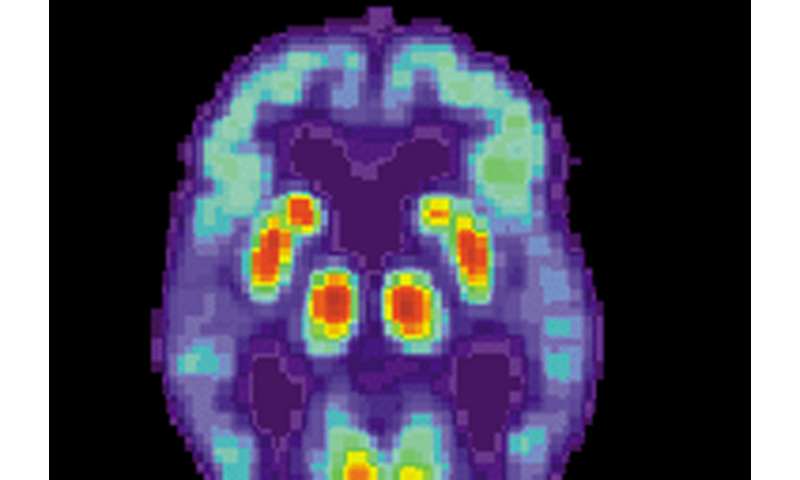
PET scan of a human brain with Alzheimer’s disease. Credit: public domain
A proof-of-concept study conducted in a mouse model of Alzheimer’s disease offers new evidence that copper isotopes can be used to detect beta-amyloid protein deposits that form in the brains of people living with Alzheimer’s. or at risk of developing,
Different types of isotopes emit positively charged particles called positron which are detectable by positron emission tomography scanners. The copper isotope used in the study, Cu-64, lasts much longer than the carbon or fluorine isotopes currently approved for use in human subjects, the researchers report. Having access to longer-lasting diagnostic agents would make the Alzheimer’s diagnosis process more accessible to people living away from major medical centers. Any clinic with a PET scanner could receive the agents in time to use the compounds in brain scans of patients living nearby.
Researchers from the University of Illinois at Urbana-Champaign report their findings in Proceedings of the National Academy of Sciences.
The effort to develop copper-based compounds to detect Alzheimer’s disease in live patients is a complicated business, said Liviu Mirica, a chemistry professor who led the new study with researcher Hong-Jun Cho. Any laboratory-created diagnostic agent must meet several criteria.
“There is one part that binds the copper and another part that binds to these amyloid peptides,” Mirica said.
In tests with compounds created in Mirica’s lab, the team found that the copper-binding region of the molecule interfered with the amyloid-binding fragment. To overcome this problem, the researchers introduced a tiny chemical spacer between the two components. This improved their molecule’s affinity for amyloid peptides.
To be effective, however, such diagnostic agents must also be able to cross the blood-brain barrier.
“They have to be small enough and greasy enough to make it into the brain,” Mirica said. “But they can’t be too fat, because then they might not be bioavailable.”
The imaging agent must last long enough for imaging but eventually decay, leaving no potentially problematic radioactive metals in the body or brain.
When they first tested their compounds in mouse brain tissue, the researchers found that the affinity of their agents for amyloid deposits was limited. The addition of a second amyloid-binding component to the molecule improved its binding and improved its ability to pass across the blood-brain barrier.
“If we do live PET imaging of mice with and without Alzheimer’s disease, we see a statistically significant difference in signal strength,” Mirica said.
Molecule reduces multiple pathologies associated with Alzheimer’s disease
Design of a multivalent bifunctional chelator for diagnostics 64Cu PET Imaging in Alzheimer’s Disease, Proceedings of the National Academy of Sciences (2020). DOI: 10.1073 / pnas.2014058117, www.pnas.org/content/early/2020/11/23/2014058117
Provided by the University of Illinois at Urbana-Champaign
Quote: Team uses copper to visualize Alzheimer’s aggregates in the brain (2020, November 24) recovered November 25, 2020 from https://medicalxpress.com/news/2020-11-team-copper-image-alzheimer-aggregates. html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link