
[ad_1]
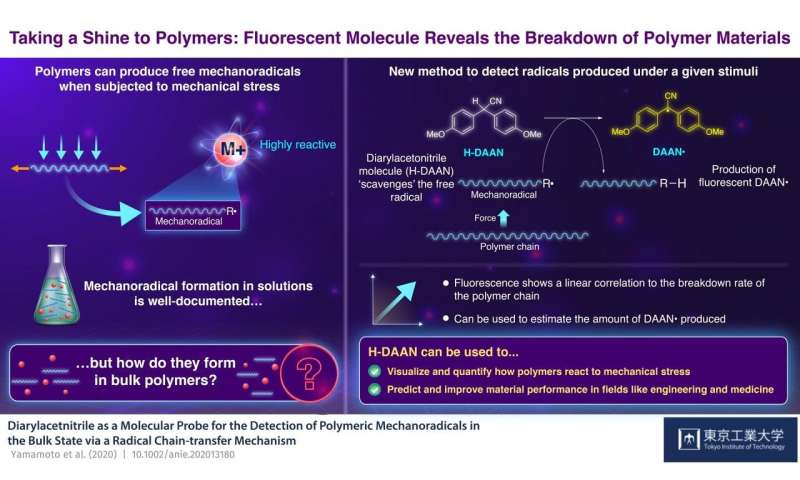
H-DAAN could function as a radical scavenger for bulk polymeric mechanoradicals and generate DAAN •, which could potentially be evaluated by EPR spectroscopy and fluorescence measurements due to their high stability towards oxygen. Credit: Tokyo Tech
Nylon, rubber, silicone, Teflon, PVC – these are all examples of man-made polymers – long chains of repeating molecular units that we call monomers. Although polymers also exist in nature (think wool, silk or even hair), the invention of synthetic polymers, the most famous of which is plastic, has revolutionized the industry. Lightweight, stretchy, flexible, yet strong and durable, synthetic polymers are one of the most versatile materials on the planet, used in everything from clothing to construction, packaging and power generation. Since the beginning of this new era in materials engineering, understanding the influence of external forces on the strength and stability of polymers has been crucial in evaluating their performance.
When subjected to mechanical stress, the weak bonds that hold some polymer chains together are overcome and one inevitably breaks. When this happens, a free radical is generated (a molecule with an unpaired electron, which is naturally unstable and very reactive, called in this case “mechanoradical”). By estimating the amount of free mechanoradicals produced, we can infer the resistance of a material to the amount of stress. Although this phenomenon is well documented, scientists have struggled to observe it at room temperature in the bulk state, because the mechanoradicals produced for bulk polymers are not stable due to their high reactivity to oxygen and other agents.
Tokyo Institute of Technology researchers led by Professor Hideyuki Otsuka have decided to accept the challenge. In their study published in Angewandte Chemie International Edition, they used a small molecule called diarylacetonitrile (H-DAAN) to capture rogue free radicals. “Our theory was that H-DAAN emit a distinctive fluorescent light when it reacts with free radicals, which could then be measured to estimate the extent of degradation of the polymer,” explains Prof. Otsuka. “The theory is simple; the greater the force exerted on the polymer, the more mechanoradical products are and the more they react with H-DAAN. This faster reaction rate results in a more intense fluorescent light, the changes of which can be easily measured.”
The researchers now wanted to see how it would work in practice. When the polystyrene (in the presence of H-DAAN) was subjected to mechanical stress by grinding, the H-DAAN acted as a radical scavenger for the polymeric mechanoradicals and bonded with them to produce “DAAN •”, which fluorescent properties. This caused a visible yellow fluorescence to appear.
“More important, probably, is the clear correlation we found between the intensity of the fluorescence and the amount of DAAN radicals generated by the ground polystyrene, as we had predicted,” says Prof Otsuka. “This means that it is possible to estimate the amount of DAAN radicals generated in the bulk system simply by measuring the intensity of the fluorescence.”
The implications of their findings are far-reaching: being able to visually quantify how materials respond to different external stimuli, they can test how polymers are suitable for various uses, depending on the mechanical stress they will have to undergo. This method could prove to be an invaluable tool for scientists and engineers as they strive to improve the performance and specificity of materials.
This exciting research sheds light on the responses of polymers to mechanical stress and lights the way forward in the search for polymer mechanoradicals!
Ultra-heavy precision polymers
Hideyuki Otsuka et al, Diarylacetnitrile as a Molecular Probe for the Detection of Polymeric Mechanoradicals in Bulk State via a Radical Chain-transfer Mechanism, Angewandte Chemie International Edition (2020). DOI: 10.1002 / anie.202013180
Provided by the Tokyo Institute of Technology
Quote: Fluorescent Molecule Betrays Breakdown of Polymeric Materials (2020, November 24) recovered November 24, 2020 from https://phys.org/news/2020-11-fluorescent-molecule-betrays-breakdown-polymer.html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link