
[ad_1]
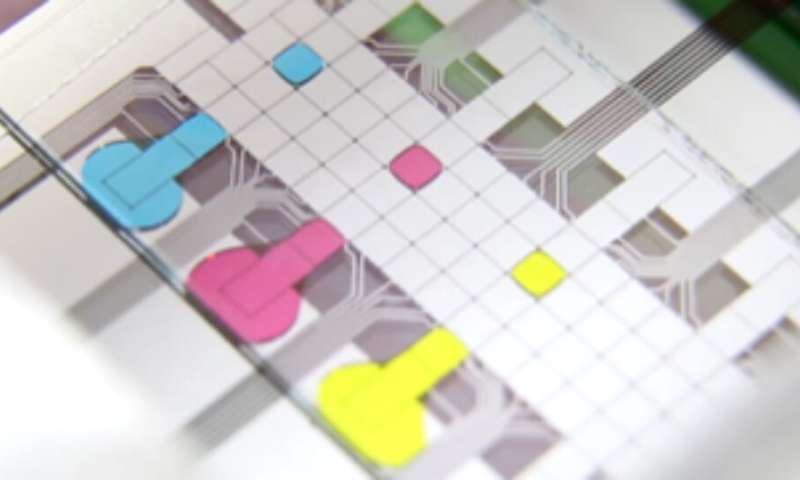
A microfluidic chip with single droplets shown in different colors. Each droplet contains material from single cells and provides it for omics analysis of single cells. Credit: Wheeler lab
Scientists can now select individual cells from a population growing on the surface of a laboratory dish and study their molecular content. Developed by researchers at the University of Toronto, the new tool will enable a more in-depth study of stem cells and other rare cell types for therapy development.
The method is the first to combine cell microscopy with omics platforms to link the physical parameters of cells that are visible to the eye, such as appearance, the presence of surface markers or cell-cell contacts, to their molecular composition.
“We give the user the ability to acquire beautiful fluorescence microscope images to learn everything that can be learned about cells growing in situ and then link that information to the cell’s genome, transcriptome and proteome,” says Aaron Wheeler. professor of chemistry and biomedical engineering at the Donnelly Center for Cellular and Biomolecular Research who led the work.
The platform is described in an article published today in the magazine Nature Communications.
Named DISCO, for Digital microfluidic Isolation of Single Cells for -Omics, the method allows researchers to select single cells in their local environment and analyze their content with DNA and protein sequencing technologies to read the cell’s DNA (genome), the RNA of transcribed genes (transcriptome) and protein molecules (proteome).
The rise in single-cell analyzes over the past five years has enabled researchers to measure tens of thousands of molecules in each cell, transforming their ability to study tissues and organs at the granular level. But these approaches lose important information about the physical characteristics of the cells and the local environment because the cells must be suspended and separated from each other before analysis.
“A revolution with single-cell homics is underway right now,” says Wheeler, Canadian research president in microfluidic bioanalysis. “But I have met people who were disappointed that they were unable to capture phenotypic information about the cell in its in situ environment.”
“And I thought we might be able to find a way to select particular cells from that population and analyze them,” he says.
DISCO is composed of a microscope equipped with a high-frequency laser and a microfluidic chip for the collection of cellular material. The microscope allows the user to acquire detailed images of the target cell before aiming the laser at it. The laser energy causes a tiny bubble to form and burst near the cell, breaking the membrane and projecting its contents in a drop onto the microfluidic chip, from where it is recovered for molecular sequencing.
“Our platform focuses on the metadata that is lost when suspending a single cell, things like the position of the cell, what were its morphological properties, who were its neighbors? These are all the things we can capture before running the single cell sequencing, ”says Erica Scott, a postdoctoral fellow in the lab who led the work alongside two PhD students. laboratory students, Julian Lamanna and Harrison Edwards.
“To our knowledge, this is the only platform that can take cells into culture and do this sort of thing,” he says.
Demonstrating the principle experiments, the researchers demonstrated the ability of DISCO to faithfully correlate omics data to single human and mouse brain tumor cells grown side by side.
But the results also highlighted the extent to which contacts between cells can affect their molecular states. The expression of a whopping 5,000 mouse genes – about a fifth of the genome – was altered in individual mouse cells that had been surrounded by human cells instead of their own relatives.
The findings could have important implications for many labs seeking to gain a better understanding of healthy and diseased human tissues, such as tumors, by culturing them in mice so they can be studied in a whole-body environment. If gene expression is similarly affected in human grafting, these changes could have ramifications for treatment development, Wheeler said.
Fortunately, DISCO may soon offer a window into cells in their natural environment as researchers are working to adapt it for tissue slice analysis. Their ultimate goal is to apply DISCO to the study of rare cell types, such as stem cells, whose regenerative potential is largely regulated by their immediate environment, to help advance new therapies.
AI methods for analyzing social networks find new cell types in tissues
Nature Communications (2020). DOI: 10.1038 / s41467-020-19394-5
Provided by the University of Toronto
Quote: Scientists can now collect individual cell contents from their local environment (2020, November 11) retrieved November 11, 2020 from https://phys.org/news/2020-11-scientists-scoop-contents-individual- cells.html
This document is subject to copyright. Apart from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link