
[ad_1]

Figure 1. Comparison of overvoltages at 10 mA cm – 2 with recently reported HER catalysts under both acidic and alkaline conditions. Credit: Professor Jong-Beom Baek, UNIST
Researchers around the world are actively working to accelerate the development of new catalysts that can significantly reduce the costs of producing hydrogen. Numerous innovative catalysts have been reported, but their expected performance is often unknown prior to implementation, and therefore more research is needed for practical use. A recent study, affiliated with UNIST, introduced a highly efficient new catalyst for hydrogen generation and its expected catalytic performance was also demonstrated.
Professor Jong-Beom Baek and his research group at the School of Energy and Chemical Engineering at UNIST have successfully developed a new hydrogen catalyst for water splitting, which consists of uniformly distributed ruthenium (Ru) nanoparticles and anchored on the surface of multi-walled carbon nanotubes (MWCNTs) or (Ru @ MWCNT). The research team also evaluated the catalytic performance of Ru @ MWCNT. The results indicated that the Ru @ MWCNT catalyst is superior in many ways to commercial Pt / C catalysts. The new catalysts are simple to synthesize and can be mass-produced, according to the research team.
‘In addition to introducing highly efficient and stable catalysts that surpass the characteristics of existing materials, this study aims to evaluate the catalytic performance of catalytic electrodes, which is an essential part of commercialization,’ says Professor Baek.
Hydrogen is the most abundant element, accounting for 75% of the universe, and has been regarded as an efficient and environmentally friendly energy source for the future. Currently, most hydrogen is produced from fossil fuels, such as natural gas, and this often releases carbon dioxide (CO2) emissions in the process. Alternatively, the process of using electricity to split water into hydrogen and oxygen has been suggested, but this requires the use of expensive catalysts, such as platinum.
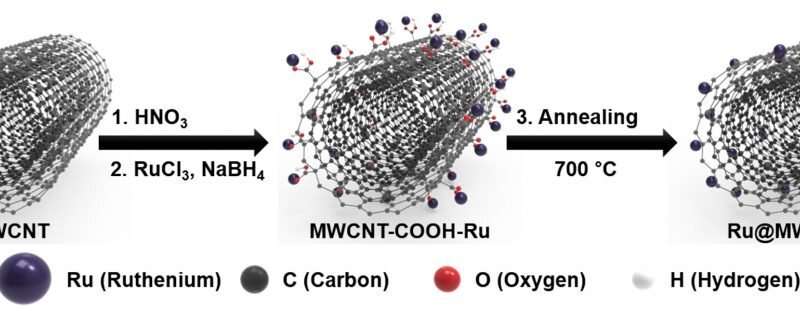
Figure 2. Schematic illustration of the process steps for the formation of the Ru @ MWCNT catalyst. Credit: Professor Jong-Beom Baek, UNIST
Therefore, Professor Baek’s team has been constantly developing catalysts that are not only superior in performance to traditional platinum catalysts, but have lower production costs. The Ru @ MWCNT catalyst exhibits superior electrochemical properties over the previously announced metal-organic catalysts. The catalyst demonstrates excellent HER performance with low overvoltages (see Figure 1), exceptional durability and high turnover rates in both acidic and alkaline conditions.
Ru @ MWCNT catalysts assume the structure, in which ruthenium (Ru) nanoparticles are uniformly distributed and anchored on the surface of multi-walled carbon nanotubes (MWCNTs). Thanks to the smaller particle size distribution and uniformity of the particles, it shows excellent HER performance and for this a production process has also been developed.
“With the existing method of combining Ru and CNT, there is a tendency for Ru particles to stick together and constantly increase the size of the agglomerate during heat treatment,” says Do Hyung Kweon (Combined MS / Ph.D. Of Energy and Chemical Engineering, UNIST), the first author of the study. ‘We suppress this agglomeration of particles by introducing’ Ru salt ‘and’ -COOH ‘and this allowed for the uniform distribution of the Ru nanoparticles on the surface of the MWCNT. “
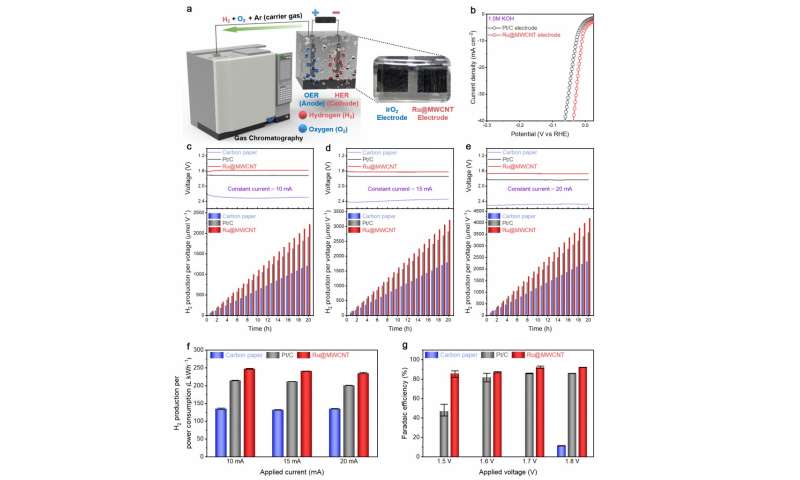
Figure 3. Evaluation of HER’s performance in actual water splitting. Credit: Professor Jong-Beom Baek, UNIST
In order to accurately determine the performance of the new catalyst, Professor Baek conducted the HER performance assessment in the construction and analysis of the water splitting system, as well as measuring the existing overpotential. Their results show that Ru @ MWCNT produces 15.4% more hydrogen per energy consumption than commercial Pt / C and Faradaic efficiency (92.28%) is higher than Pt / C (85.97%) .
‘Previous studies of hydrogen catalysts focused on evaluating the catalytic performance themselves and were not adequate to address the construction and analysis of the water splitting system,’ says Professor Baek. “This study is significant in that it can predict the actual applicability of HER.”
The results of this research were published in Nature Communications.
Design of water splitting catalysts using waste yeast biomass
Do Hyung Kweon et al. Ruthenium anchored on a carbon nanotube electrocatalyst for the production of hydrogen with greater Faradaic efficiency, Nature Communications (2020). DOI: 10.1038 / s41467-020-15069-3
Provided by Ulsan National Institute of Science and Technology
Quote: New electrocatalyst for the production of hydrogen with greater faradaic efficiency (2020, November 19) recovered on November 19, 2020 from https://phys.org/news/2020-11-electrocatalyst-hydrogen-production-faradaic-efficiency.html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link