[ad_1]
Scientists from EPFL and the Friedrich Miescher Institute used cryo-electron microscopy to explain how a DNA-sensitive biomolecule that is key to our innate immune response is inactivated when it comes into contact with the cell’s DNA.
One biomolecule that has garnered considerable attention in recent years is cGAS, a “DNA sensor” that is involved in kickstarting immune responses in the body. Specifically, when a pathogen is infecting a cell, cGAS detects its DNA and initiates a cascade of biochemical reactions that activate the body’s so-called “innate” immune system – the first line of defense of our immune system.
One of the main scientific mysteries surrounding cGAS has been how it can distinguish self from non-self DNA. But even more disconcerting is the fact that cGAS also exists inside the cell nucleus, where all the genetic material is stored.
Now, scientists from EPFL and the Friedrich Miescher Institute in Switzerland have revealed an interesting mechanism for how nucleosomes, the building units that enclose DNA within the nucleus, bind and inactivate cGAS. Published in Nature, the collaborative work was led by EPFL Professor Andrea Ablasser and IMF scientist Nicolas Thomä and provides new insights into the biology of this crucial molecule for immunity.
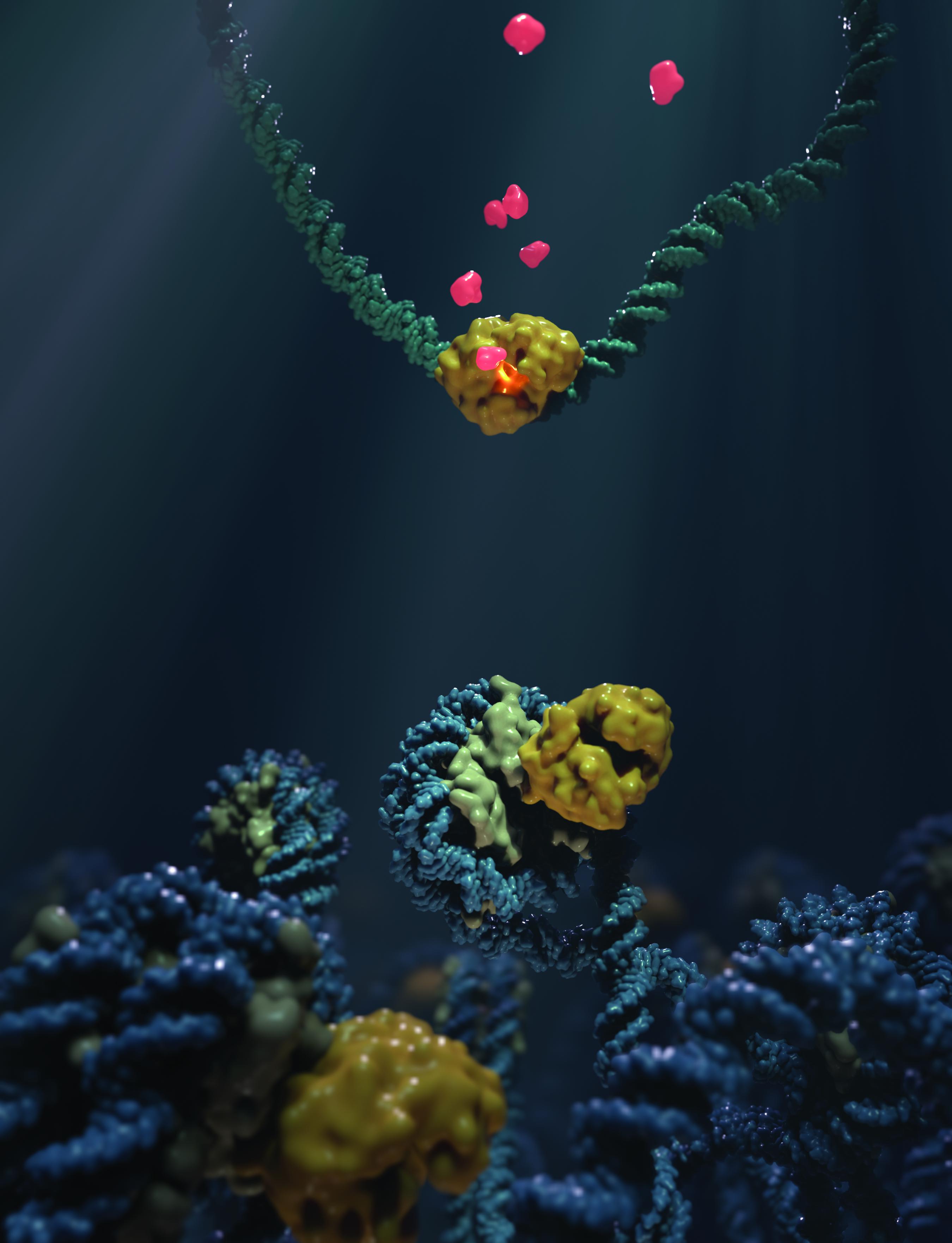
A nucleosome is made up of a string of DNA wrapped around pairs, or “dimers”, of proteins called histones, like a string around a coil. The mixture of histones and DNA is called “chromatin”.
Previous studies have found that when cells are treated with the anticancer drug aclarubicin, some histones are “mobilized” by chromatin. At the same time, cGAS is also being mobilized, which led the researchers in this study to conclude that cGAS could somehow associate with the nucleosome in the cell. The idea was finally confirmed with biochemical tests that showed that the two can actually interact.
To further understand this phenomenon, the scientists used cryo-electron microscopy to see, on a structural level, how nucleosomes bind to cGAS. By studying the complex formed by cGAS and nucleosomes with a resolution of 3.1 Ångstrom (0.31 nm), they found that cGAS binds to the so-called “acid patch” of the nucleosome; a negatively charged platform that commonly binds proteins. Once bound there, both the histones and the nucleosomal DNA “trap” the cGAS in a state where it is unable to engage or detect DNA, and is therefore functionally inactivated.

To validate their findings, the scientists introduced mutations in the cGAS-acid patch interface, which effectively prevented the cGAS from binding to the nucleosome. This was enough to remove the nucleosome blocking effect on cGAS and triggered a strong immune response in living cells.
The work offers the first structural clue to how chromatin interferes with cGAS activity. It reveals that the structural basis of cGAS interaction with chromatin provides scientists with a mechanism for how cGAS can discriminate between the DNA of its own cell and that of foreign pathogens.
Other collaborators
- University of Basel
- EPFL Institute of Chemical Sciences and Engineering (ISIC)
.
[ad_2]
Source link