
[ad_1]
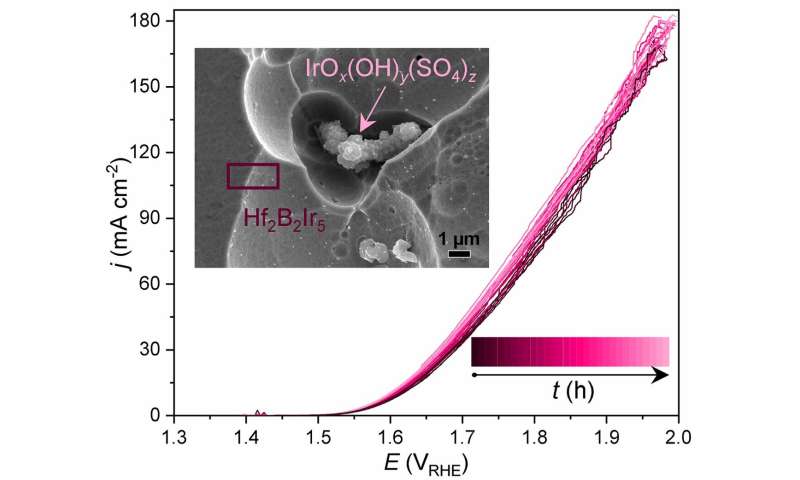
OER performance of the Hf2B2Ir5 anode material, represented by linear scan voltammograms measured during the long-term chronopotentiometry experiment (0.1 M H2SO4, j = 100 mA cm-2, t = 0 … 240 h). Box: morphology of the Hf2B2Ir5 material below. Credit: © MPI CPfS
Water electrolysis is an electrochemical way of producing hydrogen, which is regarded as one of the future energy-carrying molecules. Therefore, looking at the numerous advantages of proton exchange membrane electrolysis over the classic alkaline variant, its efficiency and large-scale applicability is of enormous importance nowadays. However, the slow kinetics of the anodic oxygen evolution reaction (OER) limits the overall electrolysis process and requires an active and stable electrocatalyst.
This need inspired scientists from the departments of Metal Chemistry and Physics of Related Matter of MPI CPfS together with the Fritz-Haber-Institut in Berlin to employ their long-standing experience in the chemistry of intermetallic compounds, in the electronic characteristics of solid matter. and in electrocatalysis to take a step forward. forward in this challenging direction. As a result of fruitful teamwork, the concept of cooperative phases with different stability under OER conditions has been successfully demonstrated with the intermetallic compound Hf2B.2Ir5 as a self-optimizing electrocatalyst for OER.
Based on the chemical bond analysis, the intermetallic compound Hf2B.2Ir5 has a type of crystal structure cage: the two-dimensional layers of B.2Ir8 the units are interconnected by two- and three-center Ir-Ir interactions with the polyanionic structure and the hafnium atoms are hosts in such anionic cages. The characteristics of atomic interactions are reflected in the electronic structure of Hf2B.2Ir5 and its chemical behavior under OER conditions.
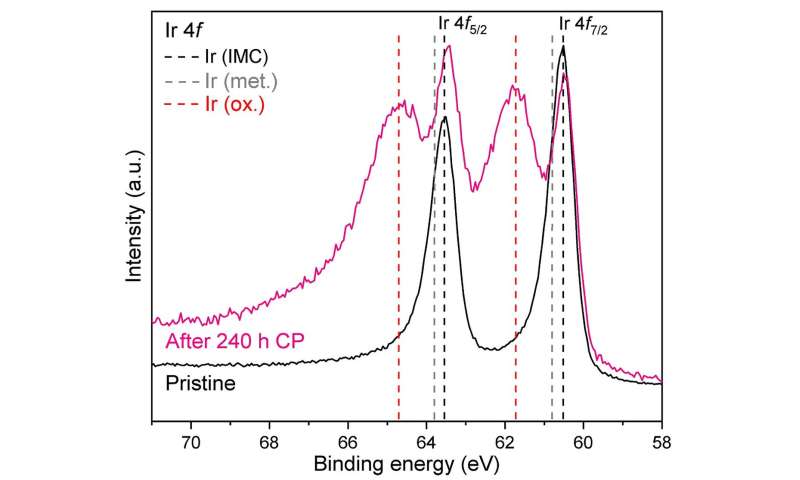
Ir 4f core levels in Hf2B2Ir5 material: pure state (black) and after 240 h of chronopotentiometry at a current density of 100 mA cm-2 (pink). The reference lines are drawn for Ir 4f in intermetallic Hf2B2Ir5 (dashed in black), elementary Ir (dashed in gray) and IrO2 rutile (dashed in red). Credit: © MPI CPfS
The initial electrochemical OER activity of Hf2B.2Ir5 sustains during continuous operation at processed current densities of 100 mA cm-2 for at least 240 h and places this material among the state-of-the-art Ir-based electrocatalysts. The harsh oxidative conditions of OER activate the limited changes to the surface of the uncontaminated material and consequently the electrochemical performance is related to the cooperative work of the Ir-terminated surface of the ternary compound itself and the agglomerates of IrOX(OH)Y(SO4) z particles.
The latter are formed mainly due to oxidation of the secondary phase HfB4Ir3 and oxidation near the surface of the studied compound. The presence of at least two OER-active states of Ir, originating from Hf2B.2Ir5 under OER conditions, it was confirmed by XPS analysis. The experimental data (electrochemical results, characterization of the material by mass and surface sensitive methods, elemental analysis of the electrolyte used) are consistent with the chemical bond analysis. The illustrated concept of cooperative phases with different chemical stability under OER conditions can be explored with other systems and offers a prospective knowledge-based way for the discovery of novel effective OER electrocatalysts.
An electrocatalyst for the oxygen evolution reaction in water splitting
Ana M. Barrios Jiménez et al, Hf2B2Ir5: A Self-Optimizing Catalyst for the Oxygen Evolution Reaction, ACS Applied Energy Materials (2020). DOI: 10.1021 / acsaem.0c02022
Provided by Max Planck Society
Quote: Electrochemical evolution of oxygen on Hf2B2Ir5 electrode material (2020, November 11) recovered November 11, 2020 from https://phys.org/news/2020-11-electrochemical-oxygen-evolution-hf2b2ir5-electrode.html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link