
[ad_1]
A new study finds that diamonds can actually form at room temperature, under the right pressure.
Image credit: Shutterstock
Diamonds are highly coveted around the world, forming naturally in the Earth’s mantle under extreme temperatures and pressures for billions of years. Now, a team of scientists from the Australian National University (ANU) and the Royal Melbourne Institute of Technology (RMIT) University have reported a means of making room-temperature gems in minutes.
Beauty aside, diamonds are extremely useful materials, especially in the field of technology, due to their extreme hardness, high thermal conductivity and quantum optical properties. To overcome the problems of availability and cost, the first synthetic diamonds were produced in the early 1950s for industrial use.
Although they are a faster means of obtaining gems, these synthetic processes still require high pressure and temperature to transform elemental carbon into crystalline diamond; this is because the energy required to overcome the high kinetic barrier between the phases of the carbon material is extremely high.
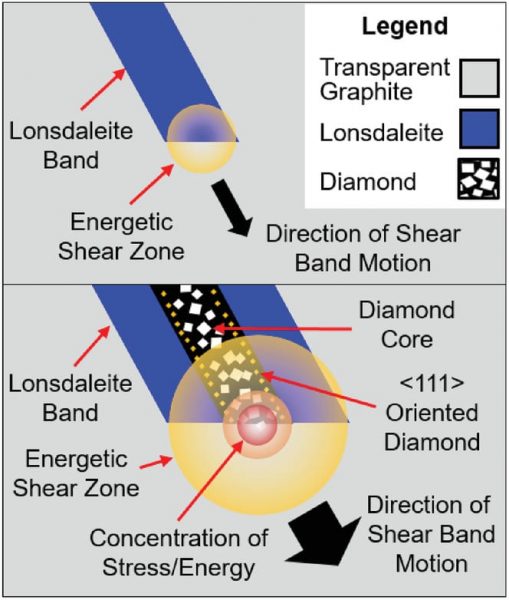
In their current study recently published in Small, the team of researchers led by Professor Dougal G. McCulloch reported the formation of nanocrystalline diamond and lonsdaleite – a less common form of carbon with a hexagonal crystal structure – at room temperature using a pressure of 80 GPa. This is a substantial improvement as most procedures require temperatures of around 1400 ° C.
The “twist” comes from how the pressure was applied. Glassy carbon was used as a precursor material and was compressed into diamond anvil cells at room temperature. “Glassy carbon is a non-crystalline form of carbon which, like graphite, is predominantly sp2 bonded, but unlike graphite, it contains overlapping and randomly oriented layers to form an opaque, isotropic material, “they said.
Using transmission electron microscopy (TEM), the researchers analyzed intact samples recovered from the cells, which they said allowed them to provide a more complete understanding of the nature and relationship between the phases present in the recovered material. “This is similar to sampling a rock core instead of crushed dust samples, e [allowed] to study the relationships at the atomic level between the arrangement of the crystallographic phases present and the non-uniform stress field present in the diamond anvil cell, “they wrote.
After compressing the glassy carbon and analyzing the samples, three distinct phases of the carbon were observed: graphite, diamond and lonsdaleite.
“It is generally thought that diamond and lonsdaleite are formed through different transformation paths that involve the sliding of the graphene layers […] to achieve proper stacking when the layers are compressed together, “the authors stated. This shifting of the atoms required to form their respective crystalline shapes is generally achieved by heating the sample to minimize the kinetic barrier that exists between the phases.
The presence of nanocrystalline veins of diamond and lonsdaleite in their samples – miraculously obtained without heating – could only be explained by the presence of high pressure and shear deformation, which results in a torsional and sliding force that helps to overcome the phase change.
“We anticipate that our observation of shear-induced phase transformations in carbon has widespread implications in a number of fields including materials science, geology and planetary science, where this mechanism can lead to phase changes in other solids.” , the authors concluded. This could also have implications in terms of where the diamond is likely to be found, both on Earth and on other planets, as the conditions for its formation have just gotten a little wider.
Reference: Dougal G. McCulloch, et al., Investigation of Room Temperature Formation of the Ultra – Hard Nanocarbons Diamond and Lonsdaleite, Small (2020). DOI: 10.1002 / smll.202004695
Source link