
[ad_1]
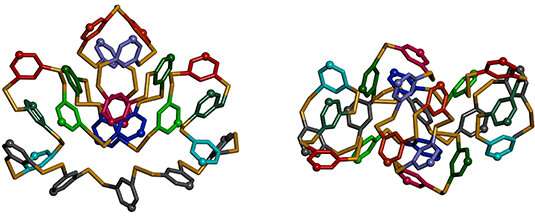
Two views of the main chain of the crystal structure of a perfectly unimolecular 23mer that forms spontaneously from a single monomer. Credit: Huc Group
The synthesis and self-organization of biological macromolecules is essential for life on earth. Chemists at the Ludwig Maximilian University of Munich now report the spontaneous emergence of complex ring-shaped macromolecules with low degrees of symmetry in the laboratory.
Monomers, molecules made up of multiple repetitive subunits that may or may not vary in their chemical structure, are classified as macromolecules or polymers. There are examples in nature, including proteins and nucleic acids, which are the heart of all biological systems. Proteins not only form the basis of the structural elements in cells, but also act as enzymes, which essentially catalyze all the myriad of chemical transformations that take place in living systems.
In contrast, nucleic acids such as DNA and RNA serve as informational macromolecules. DNA stores the cell’s genetic information, which is selectively copied into RNA molecules that provide the blueprints for protein synthesis. In addition, long chains made up of sugar units provide energy reserves in the form of glycogen, which is stored in the liver and muscles. These different classes of polymer molecules all have one thing in common: they spontaneously fold into characteristic spatial conformations, such as the famous DNA double helix, which in most cases are essential for their biochemical functions.
Professor Ivan Huc (Department of Pharmacy, LMU) studies aspects of self-organizing processes that allow macromolecules to adopt defined folded shapes. The molecular structures found in nature provide him with models, whose properties he tries to reproduce in the laboratory with unnatural molecules that are neither proteins, nor nucleic acids or sugar-like. More specifically, it uses the tools of synthetic chemistry to elucidate the principles behind self-organization, building molecules that are expressly designed to fold into predetermined shapes. Starting with the monomers his group has developed, he sets out to produce what he calls “foldamers,” assembling the monomers one by one to generate a folded macromolecule.
Structures with low degrees of symmetry
“The normal way to get the complex structure of proteins is to use different types of monomers, called amino acids,” says Huc. “And the normal method to connect several amino acids in the correct order you connect them one by one.” The amino acid sequence containing the folding information that allows different protein sequences to bend in different ways.
“But we discovered something unexpected and spectacular,” says Huc. He and his colleagues in Munich, Groningen, Bordeaux and Berlin used organic sulfur-containing monomers to spontaneously obtain cyclic macromolecules with a complex shape, as illustrated by their low degree of symmetry, without requiring a specific sequence. Macromolecules self-synthesize, no further conditions are needed. “We have only one type of monomer in a flask and expect,” says Huc. “This is typical of a polymerization reaction, but polymers of a single monomer do not usually adopt complex shapes and do not stop growing at a precise chain length.”
To further control the reaction, the scientists also used a small host molecule or metal ion. The regulator binds inside the macromolecule in growth and causes the monomers are disposed around it. By choosing a regulator with the appropriate characteristics, the authors of the new study were able to produce structures with a predetermined number of subunits. The cyclic macromolecules showed low levels of symmetry. Some consisted of 13, 17 or 23 subunits. Because 13, 17 and 23 are prime numbers, the corresponding folded forms show low degrees of symmetry.
A model for biological and industrial processes
The interest in elucidating these mechanisms is not limited to the field of basic research. Huc and his colleagues hope their approach will lead to the manufacture of designer plastics. Conventional polymers are usually made up of mixtures of molecules of varying length (i.e. the number of monomers they contain). This heterogeneity has an impact on their physical properties. Hence, the ability to synthesize polymer chains of an exact length and / or geometry should lead to materials with novel and interesting behaviors.
In addition, the foldameri such as those which have now been synthesized show narrow structural similarities with the biopolymers. They therefore offer an ideal model system in which to study the properties of proteins. Each protein is constituted by a defined linear sequence (i.e., unbranched) of amino acids, which constitutes its “primary structure”. But most amino acid chains fold into local substructures such as helical sections or parallel strands that can form sheets. These units represent the secondary structure of the protein. The term “tertiary structure” is applied to the fully folded single chain. This in turn can interact with other chains to form a functional unit or quaternary structure.
The ultimate goal is to imitate Huc of complex biological mechanisms using structurally defined synthetic precursors. He wants to understand how, for example, enzymes bend into the correct biologically active conformation as a result of their synthesis in the cells. Molecules whose properties can be precisely controlled in the laboratory provide ideal models with which to develop the answers and maybe go beyond the enzymes themselves.
The study is published in Chemical nature.
Researchers use light to design defined molecular chains
Charalampos G. Pappas et al. Emergence of low symmetry foldamers from single monomers, Chemical nature (2020). DOI: 10.1038 / s41557-020-00565-2
Provided by the Ludwig Maximilian University of Munich
Quote: Creation of self-constructed folded macrocycles with low symmetry (2020, November 23) retrieved November 23, 2020 from https://phys.org/news/2020-11-self-constructed-macrocycles-symmetry.html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link