[ad_1]
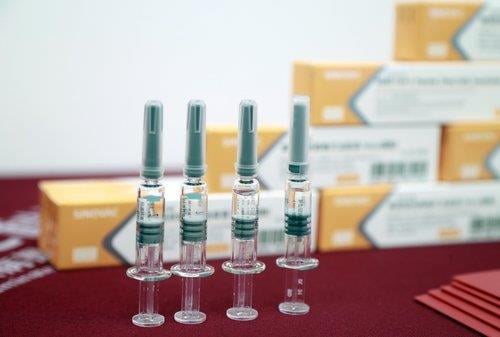
Chinese authorities are reported to plan to approve the release of 600 million doses of inactivated internal vaccines to respond to the new coronavirus infection (Corona 19) later this year.
According to Chinese media Wuhan Manbo and the Global Times on the 5th, Chinese government official Wang Jun-zhi was in the 8th West-Chinese Medicine Combined Competition held in Wuhan, Hubei Province the day before, regarding the Corona 19 vaccine. . Big news will be released within the week, “he said.
Wang Yuansa is also the deputy head of the organization of experts in vaccine development of the scientific research group of the “Joint Preventive Control Mechanism” created by the Chinese government to respond to Corona 19.
According to Wonsa Wang, as of the 2nd, there were 214 Corona 19 vaccines under development worldwide, of which 51 have entered clinical trials and 14 have entered the third clinical trial.
In China, 14 have entered clinical trials and six are in the third clinical trial, of which four are inactivated vaccines.
According to the South China Morning Post (SCMP), inactivated vaccines are a method of generating antibodies in the body using viruses that have removed their ability to replicate and have been used for the prevention of hepatitis A, polio and influenza for decades.
However, due to the limited duration of immunity and the need to inoculate a large amount over a long period of time, as well as concerns about side effects, it is estimated that it is rarely used in recent vaccine developments.
Wang said, “China has relatively good technology in inactivated vaccines” and “Since the inactivated vaccine is closest to the virus structure in its natural state, it can elicit a relatively strong human immune response and control safety.” .
Furthermore, it is an advantage that vaccines under development in the West can be distributed at 2 ~ 8 ℃ compared to having to be transported at cryogenic temperatures.
Wang said: “We have already confirmed good results of generating security and immunity at a rudimentary level.” .
“Inactivated vaccines must be produced in a biosafety level 3 (P3) laboratory,” he added. “If large-scale production is required, the laboratory biosecurity and detection technology must be world-class and China is very successful in this area.”
/ news yunhap
Ⓒ Hankyung.com prohibits unauthorized reproduction and redistribution
Source link