[ad_1]
A a group of scientists from the University of Liverpool has revealed new possibilities for the next development of clean and sustainable bioenergy.

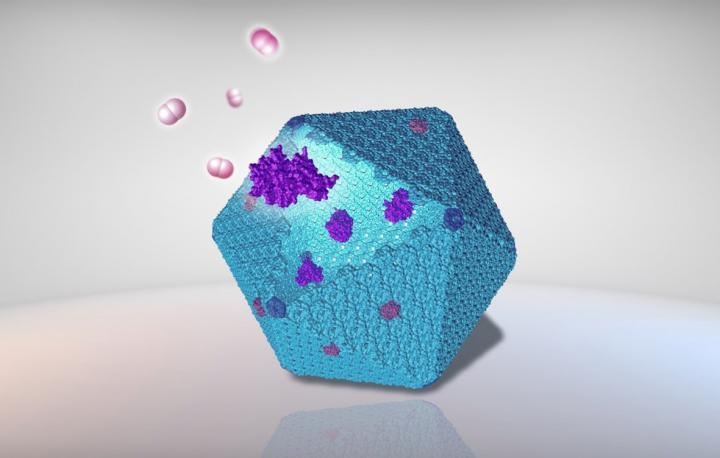
Posted in Nature Communications Journal, the study demonstrates a method by which hydrogen can be produced by reprogramming bacterial protein “cages” as nanoscale bioreactors.
The unique bacterial organelle, carboxisome, encapsulates the crucial CO2– by fixing the enzyme called Rubisco into a protein shell that looks like a virus.
Semi-permeability, naturally engineered architecture, and catalytic enhancement of carboxyisomes have led to the practical design and engineering of novel nanomaterials to integrate different types of enzymes into the shell to enhance catalytic performance.
The initial phase of the research work required investigators to install particular genetic elements in the industrial bacterium Escherichia coli (E. coli) to create empty carboxisome shells. The researchers also detected a tiny “linker” known as an encapsulation peptide that can direct external proteins into the shell.
Hydrogenases are enzymes that catalyze the production and conversion of hydrogen. The highly oxygen-sensitive trait of these enzymes has been a long-standing problem for hydrogen production through bacteria. Therefore, the researchers designed novel techniques to integrate catalytically active hydrogenases within the hollow shell.
Our newly developed bioreactor is ideal for oxygen sensitive enzymes and marks an important step towards the possibility of developing and manufacturing a bio-factory for the production of hydrogen.
Luning Liu, study leader and professor of bioenergetics and microbial bioengineering, Institute of Systems, Molecular and Integrative Biology, University of Liverpool
In association with Andy Cooper, professor at the Materials Innovation Factory (MIF) at the University of Liverpool, the team subsequently validated the hydrogen production activities of biochemically isolated nanobioreactors and bacterial cells.
The nanobioreactor achieved approximately 550% improvement in hydrogen production efficiency and greater oxygen tolerance than enzymes without shell encapsulation.
The next step in our research is to answer how we can further stabilize the encapsulation system and improve yields. We are also thrilled that this technical platform opens the door for us, in future studies, to create a diverse range of synthetic factories to enclose various enzymes and molecules for custom functions..
Luning Liu, study leader and professor of bioenergetics and microbial bioengineering, Institute of Systems, Molecular and Integrative Biology, University of Liverpool
Tianpei Li, the study’s first author and doctoral student, said: “Due to climate change, there is an urgent need to reduce carbon dioxide emissions from burning fossil fuels. Our study paves the way for the engineering of shell-based carboxisome nanoreactors to recruit specific enzymes and opens the door to new possibilities for the development of sustainable and clean bioenergy. “
The study was financially supported by the Royal Society, the Biotechnology and Biological Sciences Research Council (BBSRC), the British Council Newton Fund and the Leverhulme Trust.
The study was also conducted in association with the Center for Cell Imaging, Center for Proteome Research and Biomedical Electron Microscopy Unit at the University of Liverpool, together with scientists from Henan University and Central South University in China.
Journal reference:
Illuminated., et al. (2020) Reprogramming of bacterial protein organelles as nanoreactor for hydrogen production. Nature Communications. doi.org/10.1038/s41467-020-19280-0.
Source: https://www.liverpool.ac.uk/
Source link