[ad_1]
28 November 2020 14:43:53
 it is an iron oxide that is formed when iron reacts with water in the presence of oxygen. (Source: ISRO / NASA / JPL-Caltech / Brown University / USGS
it is an iron oxide that is formed when iron reacts with water in the presence of oxygen. (Source: ISRO / NASA / JPL-Caltech / Brown University / USGS
By Meenambika Menon
“Above the top of a high mountain, there is a beautiful moon so white.” These are a couple of lines from a poem The White Moon by Xing Yi.
Will the fact that the moon is silvery-white remain the same for years to come? Can you imagine our moon appearing reddish? The fact is that the spectrum of light reflecting on the surfaces at both poles of the moon indicates the presence of the mineral “Hematite”, in common parlance, Rust.
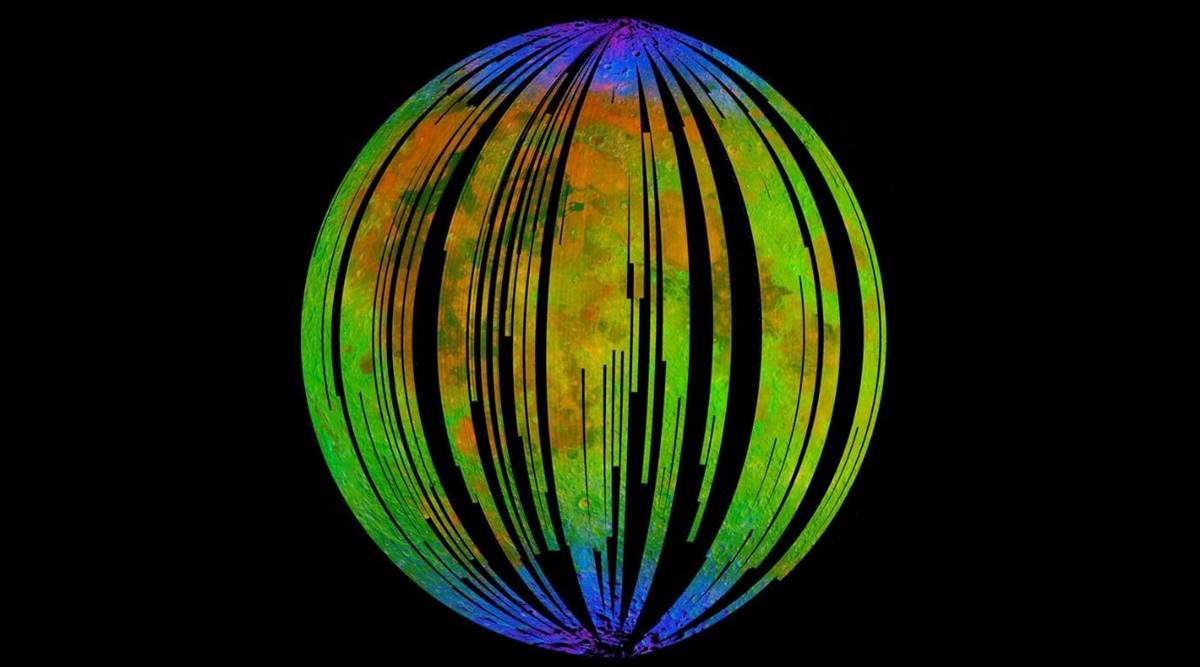
You read that right; our only natural satellite is rusting. The blue areas in a composite image from the Moon Mineralogy Mapper (M3) aboard the Indian Space Research Organization’s Chandrayaan-1 orbiter show water concentrated at the poles of the Moon. Upon entering the spectra of the rocks, the researchers found signs of hematite, a form of rust.
The blue areas in a composite image from the Moon Mineralogy Mapper (M3) aboard the Indian Space Research Organization’s Chandrayaan-1 orbiter show water concentrated at the poles of the Moon. Upon entering the spectra of the rocks, the researchers found signs of hematite, a form of rust.
What is rust? It is an iron oxide that is formed when iron reacts with water in the presence of oxygen.
To read| A science teacher explains: even the sun turns upside down
We know that the lunar crust has an average weight composition of 43% oxygen, 20% silicon, 19% magnesium, 10% iron, 3% calcium, 3% aluminum and minimal percentages of chromium, titanium and manganese. . As found by orbiter Chandrayaan I and NASA, there are traces of water found at the poles of the moon. However, the component necessary for rust to form, oxygen, is not present on the Moon as it has no atmosphere. So why does rust form on the moon?
Another puzzling factor shared by Shuai Li of the University of Hawaii is that the solar wind, a stream of charged particles flowing from the Sun, bombarding the Earth and Moon with hydrogen makes it more difficult for rust to form.
So where does this oxygen needed for rusting come from and how is the solar wind hydrogen canceled out? Believe it or not, oxygen is reaching the moon from our own land. But how?
In 2007, the Japanese orbiter Kaguya gave us the answer to this question and discovered that oxygen from the Earth’s upper atmosphere can make a spin in the wake of the Earth’s magnetic field, magnetotail, traveling 385.00 kilometers to the moon. . This finding fits data from the Moon Mineralogy Mapper instrument, or M3, which found more hematite (rust) on the near side facing the earth than on the far side.
In addition to carrying oxygen to the moon from our home planet, the magnetic tail also blocks over 99% of the solar wind during certain periods of the lunar orbit (specifically, whenever it is in the full moon phase). This results in occasional periods during the lunar cycle where rust can form.
The third piece of the puzzle concerns the presence of water. Water in the form of ice can be found in the shadowed lunar craters on the dark side of the moon which always faces away from Earth. But the hematite was detected far from it. Li’s theory suggests that fast-moving dust particles that regularly rain on the moon could release these water molecules carried off the surface, mixing them with iron in the lunar soil. Dust particles contain moisture that reacts with iron when placed on the surface. During times when the Moon is protected from the solar wind and oxygen is present, a chemical reaction could occur that induces rust.
But more data is needed to determine exactly how water and oxygen interact with iron on the moon. More data is also needed to explain another riddle: why small amounts of hematite are also forming on the far side of the moon, where Earth’s oxygen shouldn’t be able to reach.
We will have to wait a little longer to solve these mysteries of the rusting of the Moon.
(Reference source: http://www.jpl.nasa.gov, news.sky.com)
(The writer is head, high school, Shiv Nadar school.)
© IE Online Media Services Pvt Ltd
.
[ad_2]
Source link