
[ad_1]
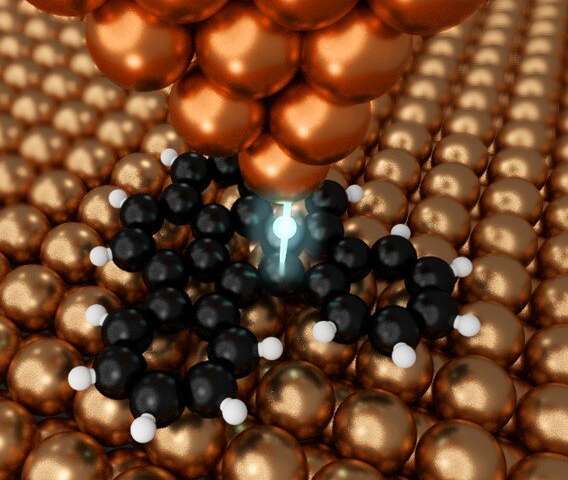
The copper probe can manipulate matter on an atomic scale. Credit: © 2020 Shiotari et al.
Nanographene is a material that could fundamentally improve solar cells, fuel cells, LEDs, and more. Typically, the synthesis of this material has been imprecise and difficult to control. For the first time, researchers have discovered an easy way to gain precise control over the fabrication of nanographene. In doing so, they shed light on the previously unclear chemical processes involved in the production of nanographene.
Graphene, atom-thick sheets of carbon molecules, could revolutionize future technology. Units of graphene are known as nanographene; these are adapted to specific functions and, as such, their manufacturing process is more complicated than that of generic graphene. Nanographene is produced by selectively removing hydrogen atoms from organic carbon and hydrogen molecules, a process called dehydrogenation.
“Dehydrogenation occurs on a metal surface such as that of silver, gold or copper, which acts as a catalyst, a material that enables or accelerates a reaction,” said Assistant Professor Akitoshi Shiotari of the Department of Materials Science. advanced. “However, this surface is large compared to the target organic molecules. This contributes to the difficulty of creating specific nanographic formations. We needed a better understanding of the catalytic process and a more precise way to control it.”
Shiotari and his team, exploring various ways to perform nanographene synthesis, have come up with a method that offers the precise control needed and is also very efficient. They used a specialized type of microscope called an atomic force microscope (AFM), which measures the details of molecules with a needle-shaped nanoscopic probe. This probe can be used not only to detect certain characteristics of individual atoms, but also to manipulate them.
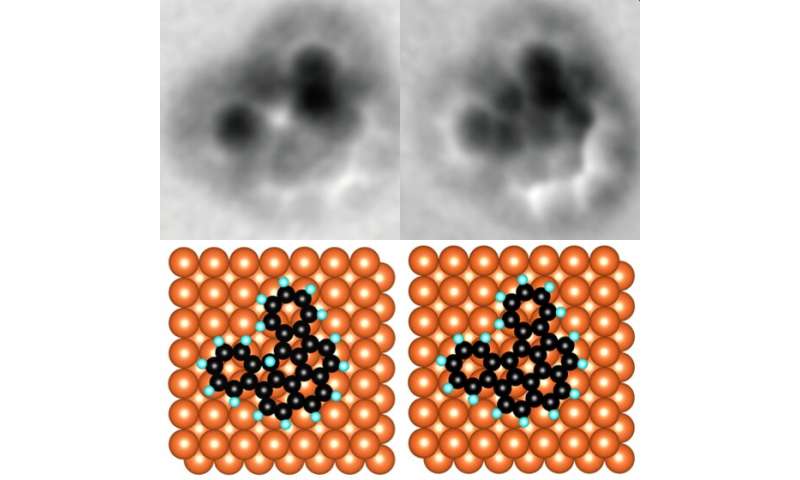
An organic molecule with an unwanted hydrogen atom (left) and the same molecule with the removed atom (right). Credit: © 2020 Shiotari et al.
“We found that the metal AFM probe could break the carbon-hydrogen bonds in organic molecules,” Shiotari said. “It could do this very precisely since its tip is so tiny and it could break bonds without the need for thermal energy. This means that we can now fabricate nano components in a more controlled way than ever.”
To verify what they were seeing, the team repeated the process with a variety of organic compounds, most notably two molecules with very different structures called benzonoids and non-benzonoids. This shows that the AFM probe in question is capable of extracting hydrogen atoms from different types of materials. Such a detail is important if this method is to be extended to a commercial means of production.
“I imagine this technique could be the best way to create functional nanomolecules from the bottom up,” Shiotari said. “We can use an AFM to apply other stimuli to target molecules, such as injecting electrons, electron fields or repulsive forces. It is exciting to be able to see, control and manipulate structures on such an incredibly minute scale.”
Graphene Research Breakthrough: Large, stable pieces of graphene produced with a unique border pattern
Akitoshi Shiotari et al, manipulatable metal catalyst for the synthesis of nanographene Nano Letters (2020). DOI: 10.1021 / acs.nanolett.0c03510
Provided by the University of Tokyo
Quote: A New and Efficient Way to Create Nanographene for Power and Display Devices (2020, November 11) Retrieved November 12, 2020 from https://phys.org/news/2020-11-efficient-nanographene-power-devices.html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link