
[ad_1]
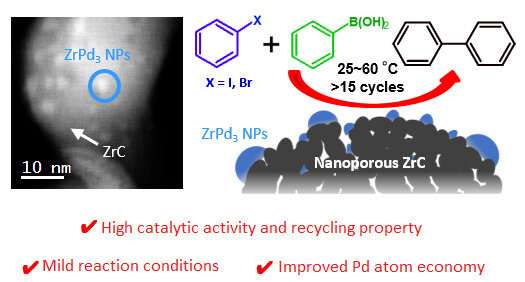
Overview of the new heterogeneous catalyst Pd-ZrC. The new catalyst consists of ZrPd3 nanoparticles grown on an inert ZrC support. Its simple manufacturing procedure, combined with its many advantages over available alternatives, make the proposed catalyst an attractive option for Suzuki cross-coupling reactions. Credit: Tokyo Institute of Technology
The Suzuki cross-coupling reaction is a widely used technique for combining organic compounds and synthesizing complex chemicals for industrial or pharmaceutical applications. The process requires the use of palladium (Pd) catalysts and, to date, two main types of Pd-based materials are used in practice as heterogeneous catalysts.
The first are “metal-charged catalysts”, which consist of Pd atoms (active sites) loaded on inert supports consisting of oxides or carbon-based materials. They are easy to prepare and offer a large surface area with active sites where the Suzuki reaction can occur. However, these catalysts degrade rapidly with use as the active sites aggregate / detach from the support. The second type are “intermetallic catalysts”: molecules of Pd and another metal. Although much more stable and effective under mild conditions, these catalysts misuse the high amounts of Pd required because few active sites actually end up being exposed to the reaction medium. But what if both types of catalysts were combined to overcome their inherent limitations?
In a recent study published in DHW catalysis, a team of scientists from Tokyo Tech, Japan, came up with a new idea for a heterogeneous catalyst. They chose nanoporous zirconium carbide (ZrC) as the support on which ZrPd is grown3 nanoparticles, which act as an intermetallic catalyst. Since both the support and the active compound have the same element (Zr), the chemical preparation of the catalyst is remarkably simple. Plus, the overall benefits go far beyond that.
First, the new Pd-ZrC catalyst is highly stable because the active sites (ZrPd3) are anchored to the nanoporous ZrC support. This strong interaction between ZrPd3 and ZrC helps improve overall catalytic stability, allowing the Pd-ZrC catalyst to be reused for more than 15 cycles. Furthermore, the exposed Pd sites do not aggregate and are dispersed throughout the support, creating an effective area much larger than the intermetallic catalysts alone. The clear distribution of ZrPd3 on the surface of the support also means that less palladium is needed for the same number of active sites than other intermetallic catalysts, a measure called the atomic economy of Pd.
Perhaps most importantly, these benefits are provided without constraints; the actual performance, i.e. the turnover rate, of the new catalyst is higher than that of commercially available compounds. Professor Hideo Hosono, who led the study, explains: “Since Pd-ZrC has both negatively charged Pd and strong electron donation capacity, our catalyst achieved high catalytic performance for the Suzuki cross-coupling reaction even at room temperature”.
Overall, the results of both theoretical and experimental analyzes conducted by the team of scientists confirm that their strategy holds great promise for the development of future catalysts, as Prof Hosono notes: “Our observations have demonstrated the effectiveness of the combination of catalysts. intermetallics with supports to improve multiple aspects simultaneously, demonstrating that we can increase the degrees of freedom in the design of heterogeneous catalysts “.
Enhancing catalysts is a practical way to reduce the economic and environmental costs associated with the synthesis of complex chemicals. Only time will tell how many new catalyst designs are inspired by the strategy adopted in this study.
A nanoscale lattice of palladium and yttrium forms a superlative carbon bonding catalyst
Yangfan Lu et al, Intermetallic ZrPd3-Embedded Nanoporous ZrC as an efficient and stable catalyst of the Suzuki cross-coupling reaction, DHW catalysis (2020). DOI: 10.1021 / acscatal.0c03416
Provided by the Tokyo Institute of Technology
Quote: A Combined Strategy in Suzuki Cross Joint Catalyst Design (2020, Dec 2) retrieved Dec 2, 2020 from https://phys.org/news/2020-12-combined-strategy-catalyst-suzuki-cross-couplings .html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no parts may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link