
[ad_1]
NASA is planning to land a crew on the Moon by 2024, and then on Mars, possibly in the 1930s. Someday we will have permanent manned bases on both worlds. Unlike short-stay initial visits, the long-term foundation will need to be self-sufficient in as many essentials as possible.
Much research has been done to prepare for the use of in situ resources (ISRU) which could help build and sustain a moon base. Now, similar ideas for Mars are catching up, with a new study, published in PNAS, which suggests a way to use the brine (salt water) found on Mars to make air and fuel breathable.
“Living off the earth” will be even more important on Mars than on the Moon, because Mars is much further away – making transportation costs (and time) correspondingly higher.
A major resource problem is how to provide enough oxygen for the Mars base crew to breathe. Mars has only a thin atmosphere, with a surface pressure less than one hundredth of Earth’s. Even worse, it is 96% carbon dioxide with only 0.1% oxygen. The Earth’s atmosphere is made up of 21% oxygen.
NASA’s Mars2020 rover Perseverance, which is already on its way to Mars, carries an experiment called MOXIE, a name invented by the Mars OXygen In situ Experiment.
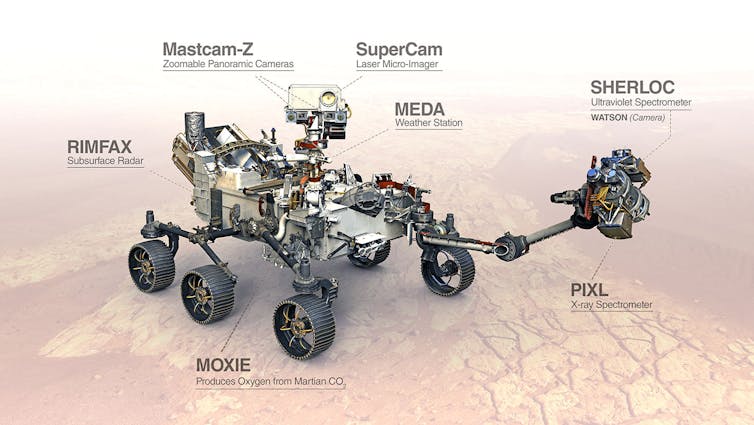
NASA
The purpose of MOXIE is to demonstrate that oxygen can be produced from carbon dioxide in the atmosphere of Mars by using electricity to split it into a mixture of oxygen and carbon monoxide, through a process called electrolysis. If it works as intended, oxygen could be collected and used to give the colonists something to breathe or as a component of fuel. Carbon monoxide would be unwanted and would be discharged back into the Martian atmosphere.
Oxygen from the Martian brine
A new way has emerged, however, that would consume 25 times less electricity to produce the same amount of oxygen. It doesn’t matter if you use solar cells or a radioactive source to generate your electricity, the available power is limited, so this is a major gain.
In the new study, a team from Washington University in the United States demonstrates how electrolysis can be used efficiently to produce oxygen and hydrogen simultaneously from brine. It turns out that when starting from a concentrated solution of magnesium perchlorate, it is relatively easy to split the aqueous component of the brine into oxygen and hydrogen using electrolysis.
This may sound exotic, but magnesium perchlorate is what brackish water appears to consist of on and near the surface of Mars, as seen for example when liquid droplets appeared on the legs of NASA’s Phoenix lander, which is landed in the far north of Mars in 2008 The Curiosity rover also found evidence of calcium perchlorate brine just south of the Martian equator.
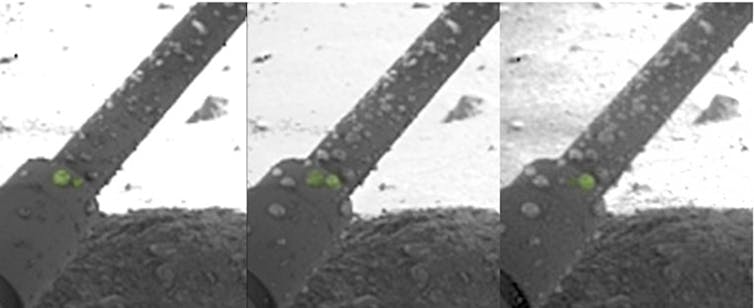
NASA / Jet Propulsion Laboratory-California Institute of Technology / University of Arizona / Max Planck Institute.
Perchlorate salts are good at collecting water from the driest atmospheres, hence the droplets on the Phoenix lander’s legs. They can lower the freezing point of a liquid as low as -70 ° C, which prevents concentrated perchlorate brines from freezing even at the low surface temperatures of Mars. There are places where the appearance of dark, moist streaks is thought to be due to the seasonal flow of brine to the surface.
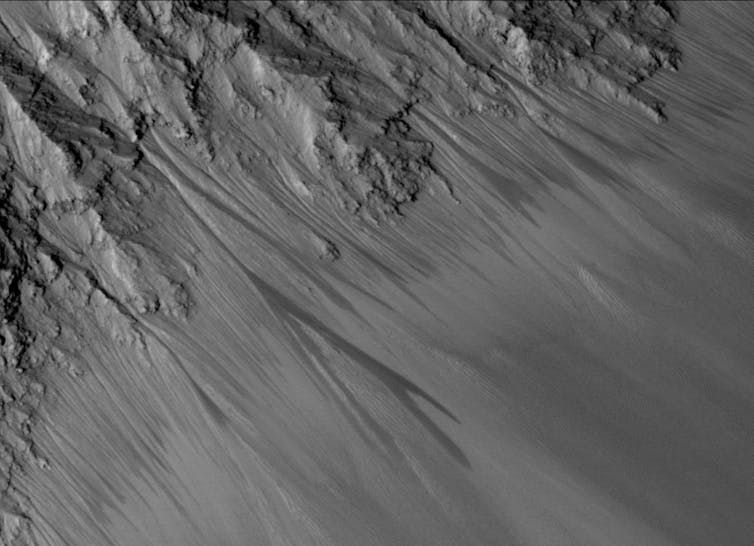
NASA / JPL / University of Arizona
If you land where there is brine available, the new study argues, you could produce as much oxygen as you want, as long as you have unlimited salt water and potency. The breakthrough in the efficiency of this perchlorate brine electrolysis has to do with the composition of the oxygen producing electrode. For this, the study used a variety of a mineral called pyrochlore, made up in this case of an oxide of lead and ruthenium. Pyrochlores have a wide range of technological applications, including, as in this case, as an “electrocatalyst” to make electrolysis faster and easier.
Read more: Water, water, everywhere – where to drink in the solar system
Practical options
It remains to be seen whether MOXIE-style electrolysis of Martian carbon dioxide or pyrochlore electrolysis of Martian brines will prove to be the most practical way to produce oxygen on Mars. The hydrogen from brine electrolysis is a bonus not obtained from carbon dioxide electrolysis and this could be used as rocket fuel, the study points out. In fact, if you want to do this, you should use oxygen as a complementary component of fuel. But at least that gives you a choice: breathe in oxygen or use it in a hydrogen plus oxygen fuel mixture.
Neither option would be available during the journey of several months to and from Mars, for which recycling solutions would have to be found, as today on the International Space Station. These would also be important on the surface of Mars.
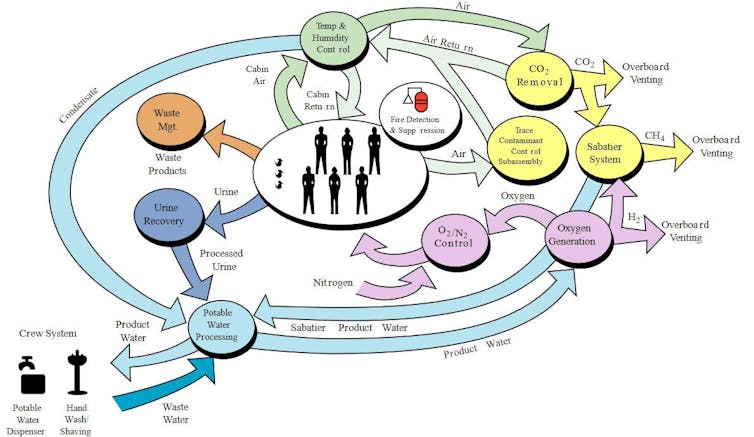
NASA
There is another way to replenish oxygen, of course, which would be to grow plants in the base of Mars. These could absorb the carbon dioxide exhaled by the crew and release oxygen through photosynthesis. Crew members could also eat some of the plants, which would be a welcome source of fresh food.
Read more: Water on the Moon: Research reveals its type and abundance, enhancing exploration plans
Source link