
[ad_1]
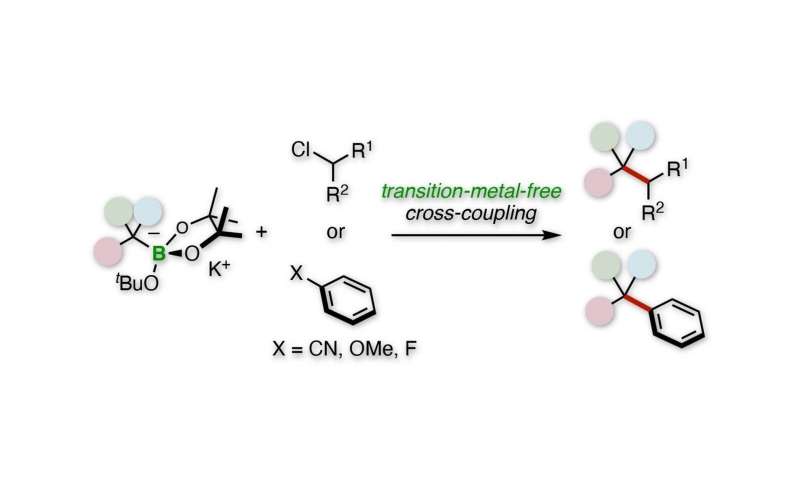
Tertiary alkylative cross-coupling of alkyl or aryl electrophiles. Credit: Kanazawa University
Pharmaceuticals, plastics and many other chemicals have transformed human life. To prepare these products, chemists often use a catalyst, often based on rare metals, at various points in their synthesis. Although rare metal catalysts are incredibly useful, their limited supply means their use is unsustainable in the long run. Synthetic chemists need an alternative.
In a study recently published in Applied chemistry, researchers from Kanazawa University report such an alternative. Their research on a broad class of chemical reactions common in pharmaceutical and other syntheses will pave the way for a more sustainable chemical industry.
The 2010 Nobel Prize in Chemistry went to researchers who used metal palladium-based catalysts to perform a common type of chemical reaction known as cross-coupling. Such catalysts work very well to synthesize so-called congested quaternary carbon centers, common in molecules used in agriculture and medicine. However, for long-term sustainability, researchers need an alternative to rare metal catalysts.
“We used benzyl organoborates to perform tertiary alkylative cross-coupling of aryl or alkyl electrophiles,” says Hirohisa Ohmiya, corresponding author of the study. “Our procedure uses no rare elements and is a direct route to the quaternary carbon centers.”
The researchers’ initial studies consisted of a tertiary benzylboronate which is activated by a potassium alkoxide base to become a benzyl anion. This anion then undergoes a cross-coupling reaction with a secondary alkyl chloride electrophile.
“The reaction is far-reaching,” explains corresponding author Hirohisa Ohmiya. “For example, the substitution of the phenyl group of boronate with various aromatic rings has been successful, and the electrophile can be a wide range of linear rings and chains.”
Subsequent studies have replaced secondary alkyl chloride with various aryl nitriles, aryl ethers, and aryl fluorides. Many of these reactions have been successful, such as those with 4-cyanopyridine and 4-fluorophenylbenzene.
A comment in Nature November 19 indicates that the COVID-19 pandemic has disrupted supply chains for various rare metals relevant to the chemical industry. Hundreds of mines and factories have been closed and many national borders are narrower than before the pandemic. A long-term solution to supply chain disruptions is to develop synthetic protocols that don’t use rare metals. The research described here is an important part of this effort and will help make chemical syntheses more sustainable for future generations.
Direct coupling of aryl halides and alkyl lithium compounds by palladium catalysis
Mitsutaka Takeda et al, Transition-Metal-Free Cross-Coupling by Using Tertiary Benzylic Organoboronates, Angewandte Chemie International Edition (2020). DOI: 10.1002 / anie.202010251
Provided by Kanazawa University
Quote: The new procedure will reduce the need for rare metals in chemical synthesis (2020, November 30) recovered on November 30, 2020 from https://phys.org/news/2020-11-procedure-rare-metals-chemical-synthesis.html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link