[ad_1]
![Pfizer, an American pharmaceutical company, announced that the preventive effect of the developed COVID-19 vaccine is greater than 90%. [연합뉴스]](https://pds.joins.com/news/component/htmlphoto_mmdata/202011/28/9d5c4a63-8811-41dd-9c6f-1e203793f21d.jpg)
Pfizer, an American pharmaceutical company, announced that the preventive effect of the developed COVID-19 vaccine is more than 90%. [연합뉴스]
Vaccination for a new coronavirus infection (Corona 19) will begin as early as the 10th of next month (local time) in the United States. Global pharmaceutical companies such as Pfizer and Modena are expected to gain emergency use approval from the U.S. Food and Drug Administration (FDA) announcing results that more than 90% of the preventative effects have been shown in Phase 3 clinical trials Expectations are also growing as the spread of the corona vaccine is expected to begin in earnest around the world after next year. However, there are still concerns about the safety of vaccines being developed at an unprecedented pace. Here are some frequently asked questions (FAQs) related to vaccines.
On the 20th (local time), US pharmaceutical company Pfizer and Germany’s Bioentech applied for emergency use authorization (EUA) for the COVID-19 vaccine under development. As a result, on the 10th of next month, the U.S. Food and Drug Administration’s (FDA) Vaccine and Biological Drug Advisory Committee (VRBPAC) will review the approval for emergency use of the Corona 19 vaccine required by Pfizer-Bioentech. If FDA approval is achieved, the vaccine will be distributed within 24 hours. Subsequently, the Centers for Disease Control and Prevention (CDC) plan to formulate a vaccination guideline and recommend that a limited initial amount of vaccination be performed for high-risk groups. The U.S. government expects Pfizer and Moderna to provide the vaccine in the quantity needed to approximately 20 million people by the end of 2020.
- What is an Emergency Use Authorization?
Emergency use approval is a procedure introduced by the FDA since 2004 to respond to public health emergencies. Drugs and vaccines can be approved more quickly on a lower level of evidence. So far, the FDA has used this authority to approve hundreds of COVID-19 diagnostic kits and treatment methods. In US history, the FDA previously administered EUAs to non-drug vaccines is just one case where the anthrax vaccine was approved by US military officials during the US anthrax terrorist incident. For this reason, some experts have wondered whether vaccines to be administered to millions of people have to go through a simple procedure. However, when Corona 19 killed more than 260,000 people in the United States alone, that rumor vanished.
Annual production of vaccines that could be approved for emergency use by US authorities next month is limited to around 40 million doses, which can be given to around 20 million people. Therefore, the World Health Organization (WHO) provides general guidelines for those who are the first to be vaccinated. However, the standards vary slightly from country to country.

Corona vaccine priority group 19 by country. Graphic = Reporter Jaemin Shin [email protected]
In the United States, four priority vaccination groups have been established. These include health care workers, essential industrial workers in the education or food industry, people with underlying illnesses, and seniors over the age of 65. In the UK, age comes first. Starting with seniors living in nursing homes and nursing home operators, they are divided into seniors over 80, over 75, over 70 and over 65. Meanwhile, the Korean government has announced that it will determine priority objectives through a meeting with a group of experts. The government said: “It will be decided based on the formulation, quantity and seasonal factors of the vaccine.”
- How the vaccine works
Both Pfizer and Moder are expected to be the first vaccines to use a new technology called mRNA (messenger ribonucleic acid), a genetic material. It differs from traditional vaccine manufacturing methods that use weakened or dead viruses. Nor is it a way to get an immune response using the virus protein. Instead, a portion of the coronavirus genetic code is injected into ultra-fine lipid cells and administered. Vaccines allow cells to produce spike proteins that protrude from the surface. So our body recognizes it as an antigen and triggers an immune response.
The vaccine developed by AstraZeneca with the University of Oxford differs from the two vaccines in that it weakens and uses adenovirus, a cold virus found in chimpanzees. When coronavirus is injected and administered to this virus, our body naturally forms an immune system.
- Are there any security issues?
Most of the Pfizer and Modena vaccine side effects reported so far are high fever, body aches, chills and headache. There have been no serious side effects, but experts agree that, in the long run, we need to look more. The FDA said it would continue strict safety checks even after the vaccine was administered.
In the case of the AstraZeneca vaccine, the controversy over clinical trial results continues. The New York Times (NYT) reported on the 25th (local time), “Experts reported that errors in the way AstraZeneca disclosed data, a number of anomalies and omissions are undermining the reliability of the results.” Previously, AstraZeneca belatedly admitted that efficacy improved due to a “dosing error” in the clinical trial process. It was planned to bring the full dose to all participants, but due to the measurement error, only half the dose was given, increasing the immune effect. It was also shown that the mode of administration for which efficacy reaches 90% has not been tested in the elderly over 55 years of age. Furthermore, it has been controversial that AstraZeneca did not disclose this fact directly, but it was disclosed by Monsef Slowe, who oversees the US government’s vaccine development project, “Operation Warp Speed”.
- How the vaccine is delivered
A “vaccine transport operation” is underway in the United States ahead of the vaccination, which will begin next month. Pfizer vaccine must be stored in cryogenic conditions below -70 degrees Celsius to maintain its effectiveness for up to 6 months. US transport company UPS has started its own dry ice production to supply Pfizer’s vaccine at low temperatures and has ordered a cryogenic freezer that can cool the vaccine to -80 ° C. Other freight companies, Fedex and DHL, would have ensured dry ice and cryogenic freezers. US internet media Axios reported that “the CDC has advised frontline medical facilities not to purchase cryogenic freezers as vaccines that do not require cryogenic storage will soon be released, but some hospitals have already started looking for freezers.”
On the other hand, the AstraZeneca vaccine can be stored, transported and handled for 6 months at room temperature of 2 to 8 degrees, which is considered the biggest advantage. However, as doubts have arisen about the vaccine’s effectiveness, it appears that it must first pass FDA approval.
![Corona 19 vaccine developed by the US pharmaceutical company Modena. [연합뉴스]](https://pds.joins.com/news/component/htmlphoto_mmdata/202011/28/02dfcffd-488f-4a81-aaf5-93bd16c471e4.jpg)
Corona 19 vaccine developed by the US pharmaceutical company Modena. [연합뉴스]
- What made ultra-high-speed development possible?
The vaccine previously approved for use in the shortest period was a mumps vaccine, which took 4 years to develop. It usually takes several years to produce a vaccine. The reason why Modena and Pfizer were able to dramatically reduce the speed of vaccine development was because of the use of the mRNA method. Previously, a procedure such as culturing raw materials in eggs was required to produce a vaccine, but mRNA vaccines do not require this process. Furthermore, before the Corona 19 outbreak, scientists were conducting experiments involving the viral genome through samples shared online.
- If you have already been infected with Corona 19.
Vaccine studies did not exclude people who had been infected with COVID-19. However, the CDC is working on a relevant guideline because there isn’t enough data yet to be sure how long the vaccine will sustain immunity.
- Is there a vaccine that works well for every person?
Experts explain that more data needs to be accumulated to answer this question. After the vaccine is approved by the FDA, it is expected that the vaccine will be customized according to CDC guidelines.
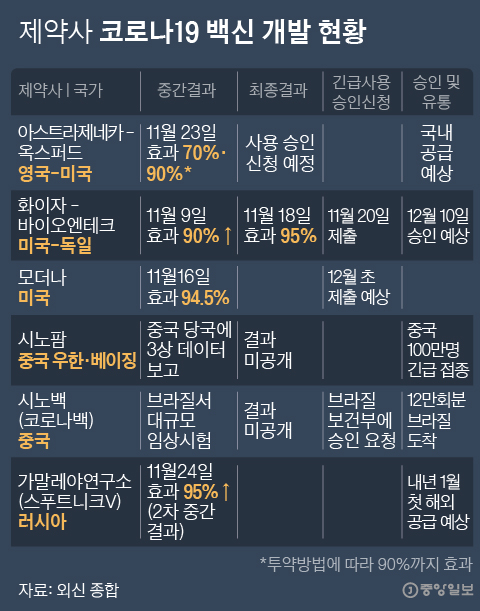
Current status of COVID-19 vaccine development by pharmaceutical companies Graph = Reporter Park Kyung-min [email protected]
- Another vaccine development situation.
In addition to the three vaccines, Russia announced on the 25th (local time) that the immune effect of the self-developed Corona 19 vaccine Sputnik V was more than 95%. Sputnik V has already been approved for use by the Russian authorities. On this day, Chinese pharmaceutical company Sinopharm also submitted an application to Chinese regulators for official approval of the Corona 19 vaccine developed by its subsidiary China National Biotechnology Group (CNBG). Once the CNBG vaccine is officially approved, China will be the second in the world to gain authority approval after Russia, allowing the vaccine to be supplied to the public.
However, in the case of the Sputnik V vaccine, controversy arose after obtaining approval from the authorities on August 11, before the results of Phase 3, which is the final phase of the clinical trial, were even released. Done. The results of clinical trials for the CNBG vaccine have not been disclosed so far.
Reporter Baek Hee-yeon [email protected]
[ad_2]
Source link