
[ad_1]
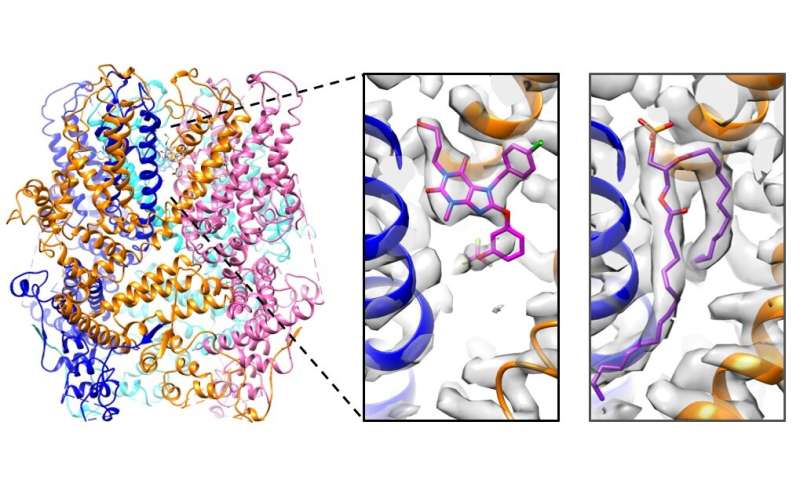
The structure of the tetrameric structure of a TRPC5 channel in complex with Pico145 (left). Each TRPC5 monomer is displayed in a different color. The structure revealed that the small drug-like molecule Pico145 can bind to each of the four pockets between the subunits (center). When the structure of TRPC5 was determined in the absence of Pico145, this pocket was occupied by a phospholipid (right). Credit: Article Authors / University of Leeds
Scientists have discovered how small drug-like molecules can regulate the activity of therapeutically relevant ion channels, and their findings could transform ongoing drug development efforts.
An important mechanism by which cells communicate with their environment is the movement of metal ions through the channels located within their cell membranes.
The new study by the University of Leeds researchers, published today in Communications biology, provides detailed information on the regulation of TRPC5 ion channels, which allow positively charged ions such as calcium, sodium and potassium to flow in and out of cells.
TRPC5 channels are considered potential therapeutic targets for the treatment of a number of conditions, including anxiety, kidney disease and cardiovascular disease.
Led by Dr Robin Bon, an associate professor of chemical biology at the School of Medicine, and Dr Stephen Muench, an associate professor of membrane biology at the School of Biomedical Sciences, the study reveals how a small drug-like molecule, called Pico145 , binds to the TRPC5 channel, thus preventing the channel from opening.
Dr David Wright, first author of the study, said, “Using cryelectronic microscopy performed at the Astbury BioStructure Laboratory, we determined the high resolution structures of the TRPC5 channel in the presence and absence of Pico145. These structures show, for the first time, how Pico145 can displace a lipid bound to each of the four TRPC5 proteins. Further studies revealed the importance of individual amino acid residues at the Pico145 binding site of TRPC5. “
Many diseases are linked to defects in ion channel function, so controlling the opening and closing of specific ion channels is a highly successful therapeutic strategy.
But drug discovery efforts are often hampered by gaps in understanding how to design drug-like small molecules to control ion channel activity.
Dr Muench said: “It amazes me that you think you understand how a small molecule can affect the activity of a protein – and then you find something unexpected.
‘Moving a lipid opens up some interesting new directions of research into how this important family of proteins functions at a fundamental level and how we can develop new therapies in the future.’
Dr. Bon said: “The opening and closing of TRPC channels is regulated by many factors, including dietary components such as lipids, minerals and antioxidants, as well as environmental toxins. The overactivity of TRPC channels is linked to a number of diseases. Therefore, Small molecules capable of blocking the opening of TRPC channels are increasingly considered potential therapeutic agents.
In fact, several pharmaceutical companies now have drug discovery programs that focus on finding new inhibitors of TRPC channels, including TRPC5.
“Pico145, which was developed by a US-based pharmaceutical company, belongs to the most potent and selective class of molecules (called xanthines) to target TRPC5 and related TRPC channels.
“In Leeds, we have worked hard to understand how xanthines regulate TRPC channel activity. Our facilities represent a breakthrough that can provide new rational approaches to the development of drug candidates that target TRPC channels.
“In addition to its relevance for drug discovery, our study also provides new insights into how physiological and dietary factors such as lipids and zinc ions can regulate TRPC channels. Thus, our work has opened up several new lines of research. “.
Adapter proteins control the gate mechanism of the ion channel
Cryo-EM structures of human TRPC5 reveal the interaction of a xanthine-based TRPC1 / 4/5 inhibitor with a conserved lipid binding site, Communications biology (2020).
Provided by the University of Leeds
Quote: Understanding Ion Channel Inhibition to Open Doors to Drug Discovery (2020, November 23) Retrieved November 23, 2020 from https://phys.org/news/2020-11-ion-channel-inhibition-doors- drug.html
This document is subject to copyright. Aside from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link