
[ad_1]
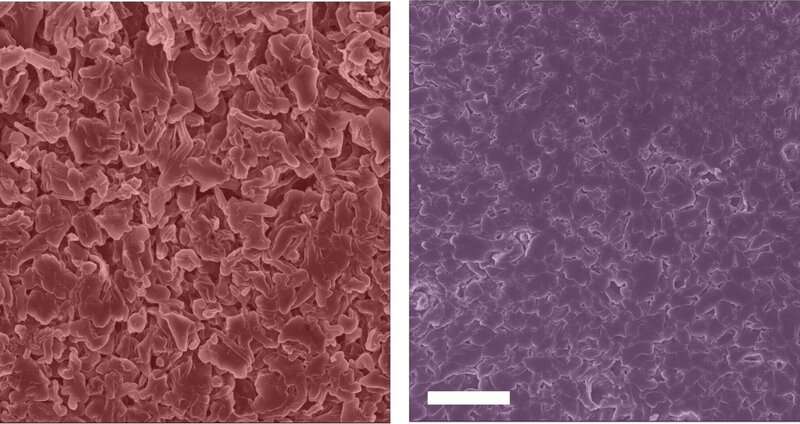
Lithium metal batteries exhibit microstructural growth when cycling into conventional lithium battery electrolyte (left). Adding potassium ions to the electrolyte modulates degradation during battery operation, preventing the growth of microstructures and leading to safer, longer-lasting batteries (right). Credit: Lauren Marbella / Columbia Engineering
Electric vehicles (EVs) hold great promise for our sustainable and energy-efficient future, but among their limitations is the lack of a long-lasting, energy-dense battery that reduces the need to do refueling on long-haul journeys. The same goes for homes during blackouts and power outages: there are still no small, efficient batteries capable of powering a home for more than one night without electricity. Next-generation lithium batteries that offer lightweight, durable, low-cost energy storage could revolutionize the industry, but there have been a number of challenges that have prevented commercialization from being successful.
A major problem is that while rechargeable lithium metal anodes play a key role in the functioning of this new wave of lithium batteries, during battery operation they are highly susceptible to the growth of dendrites, microstructures that can lead to dangerous short circuits, taking hold. fire and even explode.
Columbia Engineering researchers report today that they have discovered that alkali metal additives, such as potassium ions, can prevent the proliferation of the lithium microstructure during battery use. They used a combination of microscopy, nuclear magnetic resonance imaging (similar to an MRI), and computational modeling to find that adding small amounts of potassium salt to an electrolyte of a conventional lithium battery produces a unique chemistry at the lithium interface. / electrolyte. The study is published online today in Cell Reports Physical Science (and in the paper edition of November 18).
“Specifically, we found that potassium ions mitigate the formation of undesirable chemical compounds that settle on the surface of the lithium metal and prevent the transport of lithium ions during battery charging and discharging, ultimately limiting microstructural growth. “says PI Lauren Marbella, assistant professor of Chemical Engineering.
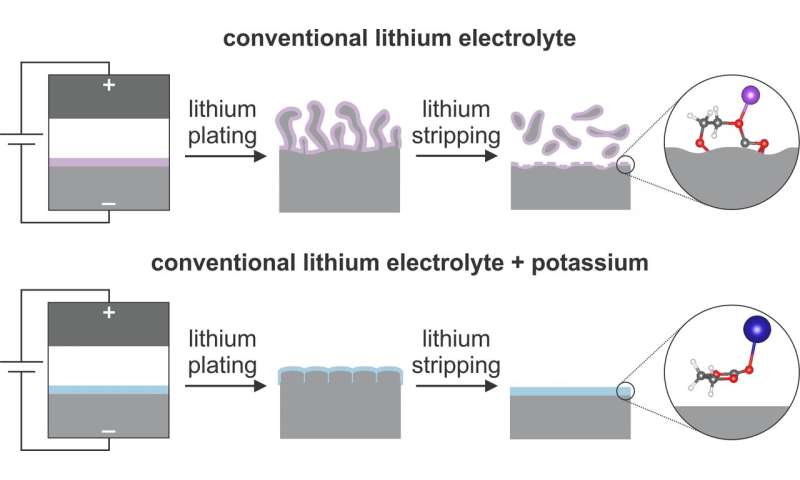
The team used nuclear magnetic resonance and computer simulations to better understand the reactivity and structure of molecules on the surface of the lithium metal anodes that could lead to better performance. Credit: Lauren Marbella / Columbia Engineering
His team’s discovery that alkali metal additives suppress the growth of non-conductive compounds on the surface of lithium metal differs from traditional electrolyte manipulation approaches, which have focused on depositing conductive polymers on the metal surface. The work is one of the first in-depth characterizations of the surface chemistry of lithium metal by NMR and demonstrates the power of this technique to design new electrolytes for lithium metal. The Marbella results were supplemented with density functional theory (DFT) calculations performed by collaborators from the Viswanathan group in mechanical engineering at Carnegie Mellon University.
“Commercial electrolytes are a cocktail of carefully selected molecules,” Marbella notes. “Using NMR and computer simulations, we can finally understand how these unique electrolyte formulations improve the performance of lithium metal batteries at the molecular level. This insight ultimately provides researchers with the tools they need to optimize electrolyte design and enable stable lithium metal batteries “.
The team is now testing alkali metal additives that block the formation of deleterious surface layers in combination with more traditional additives that encourage the growth of conductive layers on the lithium metal. They are also actively using NMR to directly measure the rate of lithium transport through this layer.
The study is titled “Leveraging Cation Identity to Engineer Solid Electrolyte Interphases for Rechargeable Lithium Metal Anodes”.
The surprising strength of liquid crystals
“Leveraging cation identity to design solid electrolyte interfaces for rechargeable lithium metal anodes.” DOI: 10.1016 / j.xcrp.2020.100239
Provided by Columbia University School of Engineering and Applied Science
Quote: New technique extends next generation lithium metal batteries (2020, November 4) recovered on November 4, 2020 from https://techxplore.com/news/2020-11-technique-next-generation-lithium-metal-batteries .html
This document is subject to copyright. Apart from any conduct that is correct for private study or research purposes, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]
Source link