[ad_1]
Input 2020.11.18 13:30 | Revision 2020.11.18 13:33
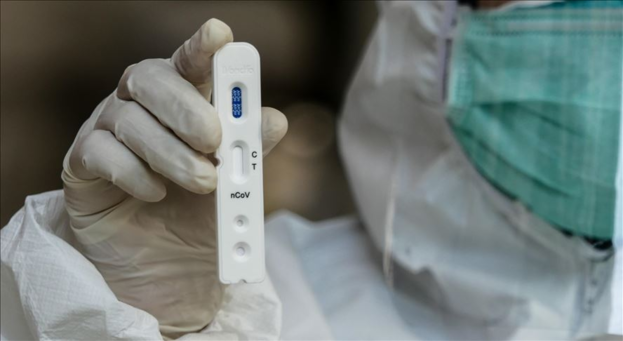
The United States Food and Drug Administration (FDA) has approved the emergency use of a diagnostic method that can easily check for a new coronavirus (Corona 19) infection at home on 17 (local time), leading media foreigners including AP and Reuters reported the same day. done.

In summary, this method, developed by the US pharmaceutical company Lucira Health, is a one-time test that detects the Corona 19 virus using a diagnostic kit.
The foreign press reported that the FDA’s decision is raising expectations that the number of corona confirmed cases19 will be able to dramatically increase the size of testing in the United States, where the number of corona confirmed patients19 is increasing exponentially.
.
[ad_2]
Source link