[ad_1]
Interview Gaëlle Legenne
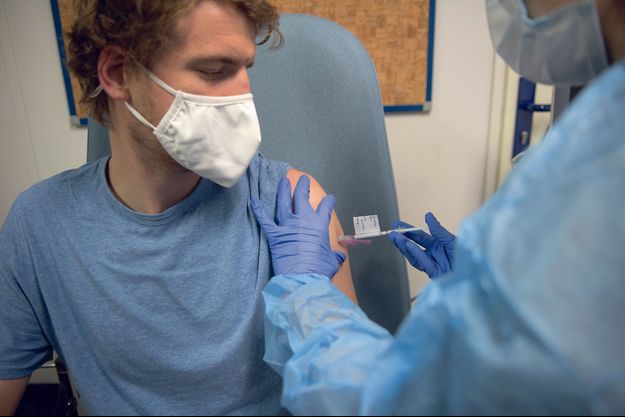
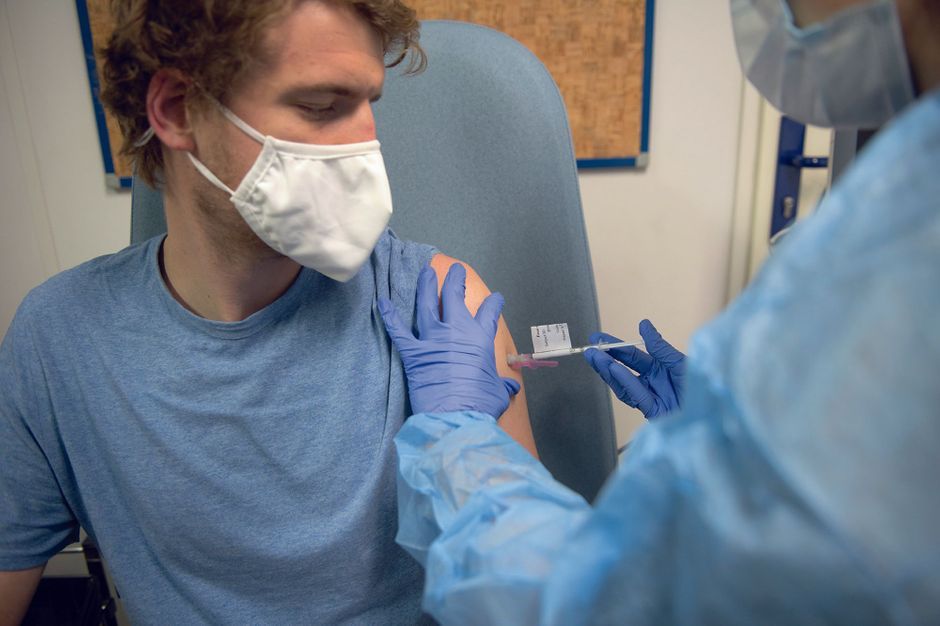
Photos from the exclusive report conducted at Cochin hospital, Paris. Virginie Clavière
Christophe d’Enfert, scientific director of the Institut Pasteur, answers Paris Match’s questions about future vaccines against Covid-19.
Paris match. Could the announcement of the Pfizer vaccine and its possible commercialization before others discourage or even interrupt other protocols in progress?
Christophe d’Enfert, Scientific Director of the Institut Pasteur. I don’t think so, no. This Pfizer vaccine is of the RNA type, based on a new technology. It is a technique that uses the body to defend itself against the virus. The price of this vaccine is likely to be high and it will likely not be easy to distribute in countries with limited resources. Vaccines based on viral vectors such as the one using the measles vector and developed at the Institut Pasteur with MSD and Themis will apparently be less expensive to produce. Like others, this vaccine was developed with the support of CEPI and Covax, coordinated by WHO. The idea is to work together for global and equitable access to vaccines for the entire world population. To take the measles vector-based vaccine example, mass production can be meaningful and affordable. Because the technique is known. The measles vaccine is already produced in large quantities. Research must be continued, because all types of vaccines will have a stake in this future campaign.
Read also: Covid vaccine: in the secret of the Russian laboratory
A phase 3 vaccine, 90% effective, how to interpret these data? Let’s think about the remaining 10%.
The 90% efficiency is extremely impressive. Very few vaccines have this effectiveness. If we take that of the flu, we are around 50%. As a reminder, the Food and Drug Administration (FDA) believed that a vaccine could be accepted as long as it confers protection for at least 50% of individuals against COVID-19. But we must be careful. In this case, these are intermediate phase 3 results. We do not yet know the duration of protection this vaccination will confer. It is too early to determine how long the body will be immune. It is encouraging, but we will have to wait for the final results. The idea of vaccination is to artificially confer collective immunity. For COVID-19, 60% of the population should be immune. It’s a big problem.
“
For COVID-19, 60% of the population should be immune.
“
Between such an announcement in phase 3 and its industrial production and commercialization, how long will it take?
Producers have already positioned themselves to produce, otherwise they have started producing. The pandemic situation has led them to anticipate the results of phase 3, so that they can vaccinate as quickly as possible afterwards. Although initially it is not necessarily produced in phenomenal quantities. However, in Pfizer’s case, they have already announced a target of one billion doses in 2021, so it may be time to market quickly. But as I said, it’s quite expensive technology that requires a first injection, then a booster after three weeks. Even if it’s fast, it will likely lead to prioritizing this vaccine over a specific type of population at risk. The question of global immunization will continue to arise.
Find our fantastic exclusive report on French vaccine volunteers in issue 3732 of Paris Match
Any reproduction prohibited
Source link
