
[ad_1]
Pfizer and BioNTech vaccine
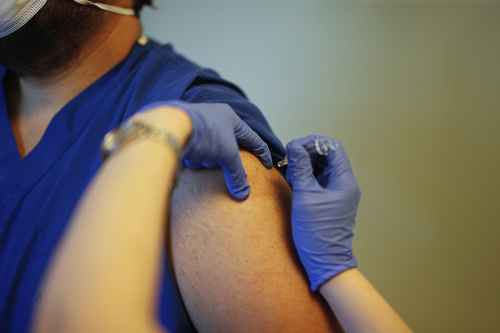
The vaccine, produced by the American company Pfizer in collaboration with the German company BioTech, reported 90% effectiveness in protecting against covid-19 regardless of gender and race. This vaccine, which showed no serious side effects in the third phase of clinical trials, appears to be the most promising. The third phase of the clinical trial involved 40,000 people. Only common side effects such as fever, headache, or body aches occurred. Some participants speak of the momentary situation as if they are looking for a monkey.
Source: TASR / AP
So far, it cannot be ruled out that the vaccine causes an allergic reaction in someone, as it is an mRNA vaccine. This is a technology that has not yet been used in any approved vaccine. Children over the age of 12, as well as people under the age of 85, also received it during testing. No information has been provided on the extent to which it can protect older people from the virus.
The 90% efficacy of the vaccine correlates with short-term protection, as it is not yet clear how long a person can be vaccinated against covid-19. We can start delivering the first vaccines ordered by the Union by the end of the year
The vaccine was also developed by AstraZeneca and the University of Oxford
British pharmaceutical giant AstraZeneca is working on a vaccine with the University of Oxford. It should be available by the end of this year. The first results of the last phase of clinical trials should be known within a few weeks. Trials around the world were suspended on September 6, when one participant developed the disease. Shortly thereafter, however, they were restored in Great Britain and later in South Africa, Brazil and Japan. Authorities determined that the disease was not related to the vaccine.

Source: TASR / AP
The Birtania vaccine combines genetic material from the surface of a coronavirus and a modified adenovirus. However, the virus is modified so that it does not cause an infection in the body. At least for Britain, it will be significantly cheaper than the Pfizer and BioNTech vaccine.
Russian Sputnik V
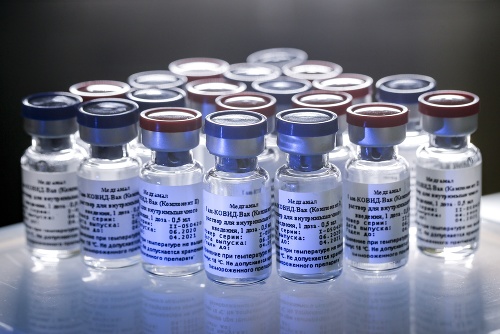
Researchers who developed the Russian Sputnik V vaccine estimate its effectiveness at 92%. More than 20,000 volunteers received at least one of two doses of the vaccine, with more than 16,000 receiving the first and second doses. A total of 40,000 people are participating in post-registration vaccine testing.
No unexpected adverse reactions were observed in subjects vaccinated with Sputnik V. – only short-term side effects were observed.
Source: TASR / AP
A virology professor at the University of Reading, England, said the data on the Russian vaccine is further good news. “Although they cover fewer cases than current data for Pfizer, as in the case of Pfizer, they confirm and develop the results of the second phase of testing. But we need to know how long it will protect and how effective it is in different age categories.” the professor said. The third phase of the clinical trial in Sputnik V has not yet started. This is a stage where not only the vaccine’s effectiveness is evaluated, but also its less common side effects.
Vaccines from China
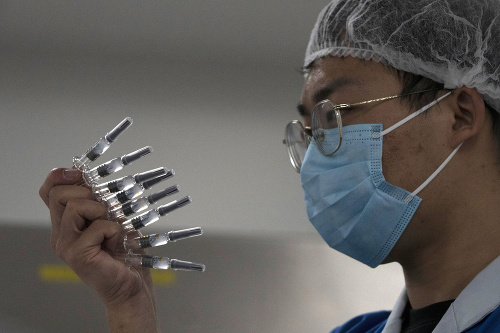
Tens of thousands of Chinese have already been vaccinated with experimental coronavirus vaccines. Two of these were developed by the Sinopharm Group and the third by Sinovac Biotech. The fourth vaccine, used by the Chinese military, was developed by CanSino Biologics.
Source: TASR / AP
Despite general warnings from experts about substances failing standard tests to assess their safety, vaccination is of international concern in China.
Beijing has not disclosed the official number of people who have received the vaccine. The third phase of the Sinovac clinical trials is underway in Brazil, Indonesia, Bangladesh and Turkey. The conclusions should be known in November.
[ad_2]
Source link