[ad_1]
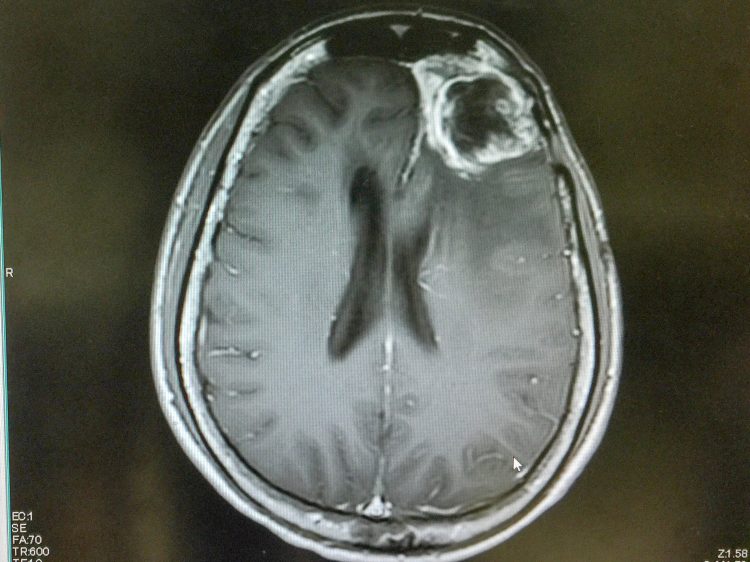
The synthetic protein nanoparticle can cross the blood-brain barrier and deliver targeted therapy to glioblastoma cells, the researchers say.
Researchers created a human serum albumin (HSA) synthetic protein nanoparticle that is able to cross the blood-brain barrier to deliver anticancer therapy directly to glioblastoma cells.
Glioblastoma is the most aggressive form of brain cancer in adults, with a median survival rate of 18 months and a five-year survival rate of just five percent. One of the biggest challenges when trying to treat glioblastoma is that it is protected by drugs given to the main circulation by the blood-brain barrier.
This barrier plays a key role in maintaining neural function, as it prevents toxins and infectious agents from entering the brain. Formed by endothelial cells with tight junctions together, the barrier uses selective transporters to strictly control what enters the brain. Unfortunately in the case of brain cancer, these properties prevent many drugs from entering the brain tissue to treat tumors.
To develop a therapy for glioblastoma that can cross the blood-brain barrier, researchers at the University of Michigan turned to HSA, one of the few molecules that can cross the barrier. Researchers led by Maria Castro, University Professor of Neurosurgery RC Schneider and senior co-author of the study, had already decided that their nanoparticle would target a factor called Signal Transducer and Activation of Transcription 3 (STAT3). STAT3 is used by cancer cells as a signal to silence the immune system, allowing them to pass safely into the brain. Using HSA as a structural building block Joerg Lahann, Wolfgang Pauli’s collegiate professor of chemical engineering and senior co-author of the study, and colleagues linked a STAT3 inhibitor and a peptide called iRGD, which serves as the latest tumor research device. synthetic protein nanoparticle.
In their study published in Nature Communications the researchers tested the nanoparticle in combination with the current standard of care for glioblastoma: focused radiation therapy. The paper states that seven of the eight mice treated with this combination achieved long-term survival and appeared completely tumor-free, with no signs of malignant tumor cells.
Furthermore, the team reported that this combination of treatments not only eradicated the primary tumor but also resulted in immunological memory; when the mice experienced a recurrence of glioblastoma, their immune systems responded by attacking the cells to prevent their regrowth, without further therapeutic drugs or other clinical treatments.
“It’s still a bit of a miracle for us,” Lahann said. “Where we expected to see some levels of tumor growth, they simply didn’t form when we got the mice. I have been working in this field for over 10 years and have not seen anything like it. “
“This is a huge step towards clinical implementation,” Castro said. “This is the first study to demonstrate the ability to deliver therapeutic drugs systemically or intravenously, which can also cross the blood-brain barrier to reach tumors.”
The researchers concluded that their synthetic protein nanoparticles could be developed to deliver other drugs and small molecule therapies to other currently “undrinkable” solid-based tumors.
Source link