[ad_1]
New research suggests that a blood test to look at the “molecular clock” of breast cancer could help monitor the growth of multiple cancers throughout the body and monitor their response to treatment.

The test, developed by British scientists, could help identify more actively growing cancers as breast cancer spreads throughout the body, helping guide the best treatment for individual patients.
The approach was developed based on new results from an innovative rapid autopsy study, the Breast Cancer Now LEGACY study.
The new study found that breast cancer spread to multiple sites follows a traceable and orderly sequence, with most new cancers in distant organs formed from cancer cells all derived from one cell in the original breast cancer.
Although further development is needed, scientists from the London Cancer Research Institute and the Royal Marsden NHS Foundation Trust believe the test would be highly sensitive and relatively inexpensive, as it does not require prior knowledge of a patient’s genetic makeup. cancer.
Secondary (or metastatic) breast cancer is the term given to breast cancer that has spread to another part of the body, such as the bones, liver, lungs, or brain, becoming incurable. Despite decades of progress, around 11,500 women in the UK still die from breast cancer each year, and nearly all of these deaths are caused by secondary breast cancer.
It is estimated that around 35,000 people in the UK are affected by secondary breast cancer. While secondary breast cancer can be controlled for some time, it currently cannot be cured and patients remain in treatment for the rest of their lives.
Relatively little is known about how and why breast cancer spreads and what can be done to treat it, in part because, as secondary breast cancer can form in sites such as the brain, liver, and bones, it can be very difficult. and painful to take. a sample of the tumor for analysis and research.
In a rapid autopsy pilot program, led by Royal Marsden’s breast surgeon Peter Barry, two women living with secondary breast cancer in London volunteered to donate their tumor tissue for research soon after their death.
The Breast Cancer Now LEGACY study, funded extensively by Breast Cancer Now and sponsored by the Royal Marsden NHS Foundation Trust and the Cancer Research Institute (ICR), has enabled surgeons, pathologists, oncologists and researchers to remove and study secondary cancers quickly after death, maintaining the integrity of key molecules within tumors (such as DNA, RNA, and proteins).
In addition to taking blood samples and biopsies from all secondary tumors, entire lymph nodes were also removed, rapidly freezing all tissue at -80 ° C. Researchers led by ICR Professor Andrea Sottoriva then studied the DNA of these cells. of secondary breast cancer, to try to better understand how these cancer cells had changed over time.
LEGACY patient 1 was a 51-year-old female who died 21 months after being diagnosed with de novo secondary breast cancer, meaning that the cancer had already spread from the breast to other parts of the body when she was diagnosed with it. first time.
LEGACY patient 2 was a 35-year-old female diagnosed with breast cancer during pregnancy. After she gave birth, it was discovered that breast cancer had spread to her bones and lungs and she died 53 months (about 4 years) after the initial diagnosis.
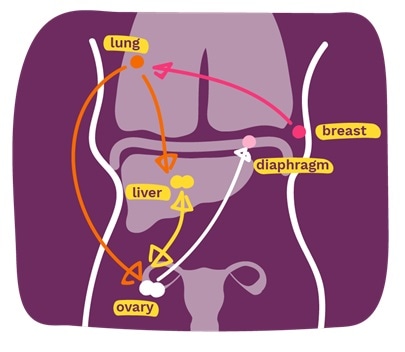
Ten of the twelve tumors found throughout patient 1’s body were ascertained by “monoclonal seeding,” which means they originated from a single primary tumor cell in the breast.
In this patient, breast cancer first spread to the lung, where a new tumor grew and evolved over time. Cancer lung cancer cells then seeded new tumors into the liver and ovaries in two separate waves. The tumor in the liver then spread to the ovary as well and subsequently began a secondary tumor in the diaphragm. Ovarian cancer also independently re-seeded the secondary tumor in the liver. Overall, only one liver sample and one ovary sample were not established by monoclonal seeding.
Monoclonal seeding was found to be the only way breast cancer spreads in the body in the LEGACY 2 patient. Researchers believe that if monoclonal seeding is the dominant way for breast cancer to spread, it could mean that secondary breast cancer monitoring is more achievable than previously thought.
Professor Sottoriva’s team then developed a new type of blood test for cancer DNA to monitor the spread of secondary breast cancer.
Over time, cells that actively grow and multiply accumulate molecular markings on their DNA, which appear in distinct patterns. The scientists found that by analyzing the cancer DNA fragments in the blood, it was possible to establish the “molecular clock” of the cancer cells from which the DNA came, which identifies how many times they had multiplied.
By analyzing these traceable “molecular clocks” and comparing the blood test with the tumors collected during the autopsy, the test built a family tree of the tumor cells and the DNA level of the tumor cells in the blood then provided information on which secondary tumors they were the most active.
However, the researchers found flaws in how cancer cells added molecular markings to their DNA in patient 2, and so more research is needed to understand how common this challenge might be before a test can be developed for clinical use.
Also, looking at data from a previous study out of eleven primary breast cancer patients whose disease had spread to the lymph nodes and for whom tissue and blood samples were available, the team confirmed that the “molecular clock” blood test mirrored the genetic makeup of the tumor samples.
The authors propose that blood tests can be used to monitor the evolution of secondary cancers over time and to monitor their response to a range of treatments, including chemotherapy, immunotherapy, or targeted therapies, as well as radiation therapy. With further development, it is hoped that the “molecular clock” blood test can also be used for early detection of relapse or spread after treatment and may also be relevant for other forms of cancer.
Our study sheds light on two of the central challenges in cancer research and treatment: cancer’s lethal ability to adapt and evolve and its tendency to spread from the initial cancer to other parts of the body.
The LEGACY study gave us a unique opportunity to analyze the genetic makeup of breast cancer after it has spread to multiple sites in the body, shedding new light on the course of cancer evolution.
We also identified a new way of understanding how cancer grows and evolves by analyzing the “molecular clock” signatures from cancer DNA in the blood.
We hope to develop a blood test to monitor the evolution of an individual patient’s cancer and, in so doing, to offer hope for effective treatment even where there has been a large spread of cancer. “
Professor Andrea Sottoriva, Director of Cancer Evolution in the new Center for Cancer Drug Discovery at The Institute of Cancer Research, London
This was a wonderful result of the collaboration with a highly innovative ICR team led by Professor Sottoriva. It points the way to potentially sampling 1 active metastatic site in a patient and then using the molecular clock signature with regular blood tests, testing and monitoring new treatments in real time.
Clearly we need to expand this test to a larger patient cohort to see how widely it could be applicable – in breast cancer patients and potentially in patients with other cancers. I am wholeheartedly grateful to the patients and their families who have so generously made this study possible. “
Peter Barry, Head of Surgical Oncology for the LEGACY Breast Cancer Study Now and Consultant Breast Surgeon at Royal Marsden, London
Dr Simon Vincent, director of research, support and influence at Breast Cancer Now, which funded the study, said:
“Studying how and why breast cancer spreads in the body is vital if we are to find a way to stop it. This groundbreaking study, made possible through selfless patient donations, helps us understand how breast cancer spreads from one part of the body to another, paving the way for further research to stop secondary breast cancer.
“Developing a blood test to continuously monitor how a patient’s cancer is changing and how they respond to treatment is an exciting step in being able to offer patients a more personalized treatment plan that could be readily adapted if a certain therapy is not. However, for this blood test to reach clinics, further testing and refinements are now needed in more people.
“With 11,500 women and 85 men dying from breast cancer every year in the UK, we need to find new ways to stop the spread of breast cancer and to effectively treat it when it does.
“Anyone concerned about the spread of breast cancer can speak to one of our expert nurses by calling our free helpline on 0808 800 6000.”
Breast Cancer Now’s ambition is that by 2050, all people diagnosed with breast cancer will live and be helped to live well.
The charity funds around a quarter of publicly funded breast cancer research in the UK and has launched its Not survived campaign in 2019, highlighting that 11,500 people still die from breast cancer each year and calling for urgent changes so that all people with secondary breast cancer can live well for as long as possible.
Breast Cancer Now thanks Walk the Walk for its generous support as one of the founding founders of the LEGACY study for secondary breast cancer.
.
[ad_2]
Source link