[ad_1]
Kathryn J. Wu – (The New York Times) – translated by Dr. Belqis Donyazad Ayashi –
The U.S. Food and Drug Administration has given the green light to the first rapid test to detect Corona virus at home – from test start to finish -, opening a potential path for more widespread testing outside of healthcare facilities, thus shortening the path to virus elimination.
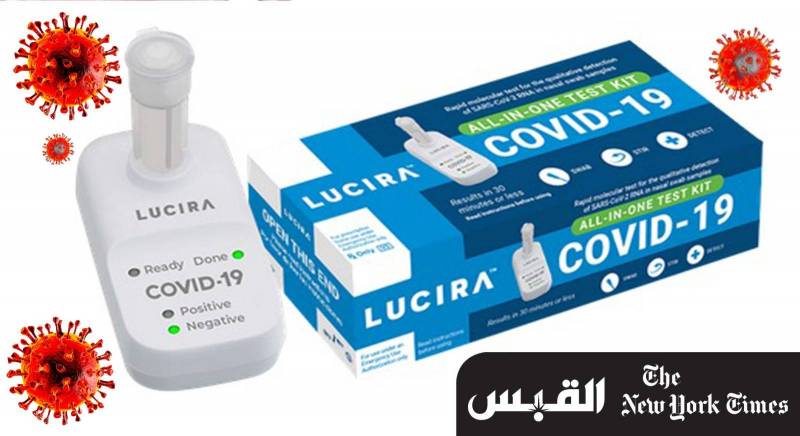
The test was developed by California-based Lucira Health, and people under the age of 14 cannot take the test on their own, but rather need someone to help them and, with a relatively simple nasal swab, the The test can deliver results in about half an hour, and the company expects the test to cost $ 50 (equivalent to 15 Kuwaiti dinars), according to the product’s website, and the device is only used once.
Doctors can also test their patients, including children under the age of 14, and are likely to provide answers during a single visit to a treatment center or pharmacy, rather than targeting a sample that is difficult to collect through the lab.
Some other tests have been approved by the FDA for collecting samples at home, which are then shipped to the lab for processing, but the Lucira test is the first test that eliminates the need for a medium.
An important step
“Today’s authorization for a comprehensive home test is an important step in the FDA’s national response to the Coronavirus,” said Jeff Shorin, director of the Food and Drug Administration’s Center for Devices and Radiological Health, adding, “Now, more Americans will be able to. They are infected with the virus by taking immediate measures, based on their findings, to protect themselves and those around them.
People who test positive for the virus should isolate themselves from others for 10 days, from the day their symptoms begin or the day they test positive for the virus, according to guidelines from the Centers for Disease Control and Prevention in the United States.
The way it works
Laboratory tests that look for Coronavirus genetic material using a technique called polymerase chain reaction, or PCR, are still considered the best standard for detecting the virus. But the new home test is based on similar principles, using a method called loop amplification reaction or LAMP. Like the polymerase chain reaction (PCR), LAMP repeatedly copies genetic material until it reaches detectable levels, making it possible to recognize the virus even when it is present at very low levels in the respiratory system. Although it is faster and less complex than PCR, it is generally believed to be less accurate.
People doing the battery-operated test should slide a swab into each of their nostrils, then dip it and mix it in a vial of chemicals.
This vial is then attached to a test cartridge that handles the sample. Within half an hour, the test cartridge will illuminate “positive” or “negative”.
Federal guidelines state that people who perform the test should report the results to healthcare professionals, who must then report to public health authorities to help monitor the spread of the virus.
“A home test for the virus would have made a difference in killing the virus,” says Omari Garner, a clinical microbiologist and diagnostic expert at the University of California.
“The news should be taken with caution, however,” noted Dr. Garner. In recent months, many experts have called for more home testing as a way to limit the spread of the virus. But others have raised concerns about the practicality of a strategy that risks relying on tests that sacrifice a degree of accuracy for convenience and a reasonable price.
Detection capability
According to the product instructions, the company’s test was able to accurately detect 94% of the infection detected by a polymerase chain reaction (PCR) based test and correctly identified 98% of healthy and not infected.
Lucira Health representatives did not immediately respond to requests for comment.
Saskia Popescu, infection prevention expert and epidemiologist at George Mason University, also warned that “home tests, despite their remarkable progress, should not be considered a panacea to help fill further gaps in coronavirus diagnoses.” adding, “We need more. Easy access and quick lab tests.”
Al Qabas readers are browsing now
[ad_2]
Source link