[ad_1]
To date, the coronavirus disease (COVID-19) pandemic has affected over 58.52 million individuals and has resulted in more than 1.38 million deaths worldwide. Second waves of pandemics are still ongoing in many countries with a huge impact on health systems and economies.
What are the non-pharmaceutical interventions that help control COVID-19?
Globally, nations struggle to effectively control the pandemic by maintaining a balance between public health and socio-economic factors. In the absence of curative therapies and preventive vaccines, most efforts are focused on “ non-pharmaceutical interventions ”, including large-scale movement restrictions or “ blockades ”, social distancing, increased sanitation of public spaces, wearing masks in public, frequent hand washing and increased testing for COVID-19.
Although many countries have implemented non-pharmaceutical interventions to control COVID-19, it is unclear how different control strategies have affected the transmission dynamics of SARS-CoV-2 in local communities.
The Nordic study analyzing SARS-CoV-2 transmission chains in sampled genomes
The Nordic countries – Denmark, Finland, Norway, Iceland and Sweden – offer a good example of geographically, socially and politically related countries whose early COVID-19 control strategies vary significantly. By analyzing the local epidemiological consequences of various control strategies, we can collect key data on the most effective approaches that inhibit transmission of the virus in communities.
This is exactly what a team of researchers from the University of Melbourne, the Peter Doherty Institute for Infection and Immunity, the University of Sydney, Australia, the Norwegian Institute of Public Health, the University of Oslo, Norway; and the University of Uppsala, Sweden discuss it in their study published on the prepress server medRxiv*.
The researchers conducted a series of analyzes on ‘transmission lineages’, ie the transmission chains of sampled genomes, from the Nordic countries. The estimated time of appearance of the transmission chains provides data on the commencement of community transmission. With the help of complete SARS-CoV-2 genomes, the research team deduced the relative frequencies of virus import and export and the dynamics of the virus transmission chain in the Nordic countries during the first 6 months of the pandemic.

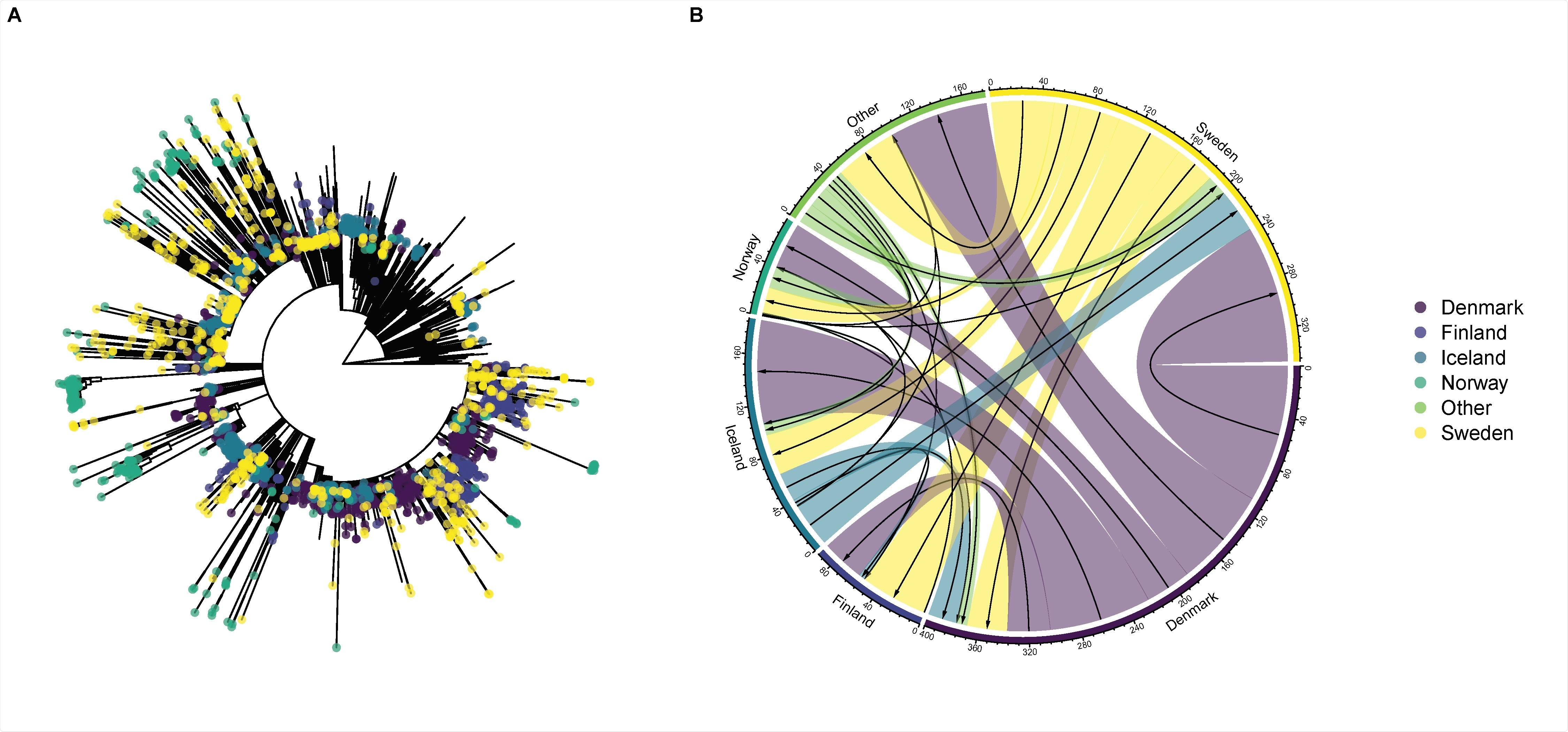
Phylogenetic tree of SARS-CoV-2 from Nordic countries and global genetic diversity. The tips are colored according to each Nordic country (panel A). Circular migration diagram of migration events between the five Nordic countries and other locations. The size of the colored arrows denotes the posterior mean number of migration events inferred from the Bayesian phylogeographic analysis. The black arrows represent the direction of migration (box B).
Sweden has more transmission chains than SARS-CoV-2 and has allowed viral spread to nearby locations
The analyzes showed that Sweden had increased the number of transmission chains with more cases and longer duration. All these characteristics have increased over time. Furthermore, Denmark and Sweden were the net exporters of SARS-CoV-2. Iceland, Norway and Finland were found to exhibit lower levels of virus export.

Number of SARS-CoV-2 genomes sampled over time for all Nordic countries and global (ie “other”) samples included in the final alignment.
The analyzes also showed that Finland was the main recipient of virus exports from Sweden, suggesting a one-way export mode between the two countries. This shows that Sweden was an epidemiological and evolutionary “refuge” that allowed the active transmission of the virus and the spread of the virus to surrounding localities. This analysis highlights the importance of genomic surveillance and demonstrates that active monitoring of the transmission chain is an important metric in the analyzes.
“This analysis highlights the usefulness of genomic surveillance where active monitoring of the transmission chain is a key metric.”
Sustained transmission chains offer greater mutational potential to the virus
A critical and little-addressed problem in this case is that when the transmission chains remain active for a long time, they offer greater opportunities for the virus to adapt to local populations and to evolve and mutate, thereby improving viral fitness.
In addition to offering greater mutational potential to the virus, sustained transmission chains also provide an epidemiological refuge for the virus to be transported to other locations, which agrees with the study’s findings showing Sweden’s highest frequency of export events among Nordic countries.
“In addition to providing greater mutational potential, sustained transmission chains also provide epidemiological ‘havens’ for the transmission of the virus to other locations.”
According to the authors, disrupting and stopping chains of transmission not only helps minimize the spread of the virus within communities, but also reduces the chances of accumulation of viral mutations beneficial for the sustenance of the virus.
Overall, this study highlights the importance of genomic surveillance and retrospective analyzes for understanding the differences in pandemic responses in terms of transmission dynamics. The study data suggests that chain-of-transmission monitoring may be a crucial metric for comparing the results of outbreak mitigation strategies.
“Overall, our study highlights the utility of continuous genomic surveillance and retrospective studies to compare and understand differences in pandemic responses to transmission dynamics.”
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and therefore should not be considered conclusive, guide clinical practice / health-related behaviors, or treated as consolidated information.
.
[ad_2]
Source link