
[ad_1]
Human trials of the Covid-19 vaccine have been underway since August. Target: 2021
What if the future of world health was played out in a prefab building located in the heart of Cochin hospital in Paris? At the end of a maze, the Cochin-Pasteur Vaccinology Center (AP-HP) doesn’t look like much. Two consultation rooms, a laboratory for the conditioning of samples, cakes in a plastic container, hydroalcoholic gel and some human warmth. Well, the cold of this first Thursday in November is stinging. The place is cramped. The corridor is crowded with researchers, assistants and volunteers. Stretched mask on the face, everyone has one goal: to advance the only trial conducted to date in France, developed last spring from the measles vaccine by the vaccine innovation laboratory of the Pasteur Institute.
Read also:“The 90% efficiency is extremely impressive for a vaccine”
This morning, in the waiting room, Erwan, 29, fools boredom with his smartphone. For reasons of confidentiality, your name, like that of the other participants, cannot be disclosed. He waited two hours to get his second injection. Investigator at INSEE, this is not his first clinical trial. Last summer she lent herself to the study of a molecule in cancer research. On October 1, listening to Inserm’s request for Covid vaccines, he registered on the Covireivac platform. It doesn’t allow him to earn a living, he is only paid, but he has the will to collaborate in a collective effort. Just like the socialist deputy from Ardèche Hervé Saulignac, 50, who has not yet been called. “We cannot imagine an economic and social life at this rate, and I see no other solution in an epidemic than to produce a vaccine, he summarizes. I have spoken about it publicly and received messages accusing me of taking part in the conspiracy. I told myself that it was more necessary than ever to wage this struggle between obscurantism and progress. I don’t know many world volunteers around me and I am surprised. “
Read also:Covid-19: two Chinese vaccines tested on one million people
Be between 18 and 55 years old, with a BMI below 30 (overweight), no medical history, not having contracted Sars-CoV-2 (Covid-19), not having a parenting plan in every twelve months, commit to use a contraceptive method during the study and, of course, read and sign an informed consent, then respect the appointment dates: all this is part of the twelve inclusion criteria of this phase 1. Erwan meets all of them and none of the twenty-five criteria of exclusion, including alcohol (within the past three years) and cigarette abuse, psychiatric disorders, lung disease, or the presence of tattoos that prevent injection site assessment. Thomas, 29, an engineer, was also selected. Wait in the waiting room. “I work in research,” he explains. The call was forwarded to my workplace, I wanted to help. My wife told me, “I wouldn’t have done that, but that’s okay.” I hadn’t thought my approach would surprise my relatives. I recently reported being in touch. I haven’t been sick. We know there’s a one in three chance of having received the placebo, but maybe I’m already protected from the first injection … “
Professor Odile Launay, specialist in infectious diseases at AP-HP and head of the clinical investigation center, passes a head, greets the participants, ensures their well-being. The atmosphere is almost familiar. “The first vaccinations began in late August, with two doses twenty-eight days apart,” he says. The immune response is measured four weeks after each injection. We know it works in animals, but the first human data, in terms of immune response, won’t be available until the end of the year. “

Volunteer for the test, Hervé Saulignac, elected Socialist of the Ardèche, in the gardens of the National Assembly, on 3 November. © Virginie Clavières / Paris Match
Explains Dr Marie Lachâtre, specialist in infectious diseases: “Among our 90 participants, there are three groups: those who receive a low dose of the candidate vaccine, those who have a high dose and, finally, those who have a placebo. This is called a “double blind” test. “At the same time as preparing the injection, remind Thomas of the rules in force:” After the injection, you must remain under observation for an hour. Severe allergic reactions, of the anaphylactic type, can occur very quickly. They are rare and agreed, but this risk requires a monitoring period, with injectable adrenaline available and the proximity of resuscitation. Thomas confirms that he has kept the thermometer and the ruler, the “kit” that allows each participant to transmit their own temperature curve as well as to measure any button that may form around the injection point. It’s red? It’s swollen? At the slightest side effect, at the least demand, the volunteers can dial, day or night, a number placed at their exclusive disposal. Thomas has never had a fever or pustules. In two weeks he will be back at the center for a checkup.
The manufacturing plants are kept secret. We just heard they’re in France.
100 meters away, the central pharmacy of Cochin hospital receives a new prescription by fax. Corinne Guérin and Jérémie Zerbit, doctors in pharmacy, are busy. “Between the moment we take the vaccine out of the freezer and the moment we inject it,” Corinne explains, “we have an hour. Not anymore. As soon as we receive the prescription, we have to go to a site, enter our codes for find out what to prepare: vaccine or placebo. We’re the only ones with this information. See that big blue binder? You won’t touch it! Contains files of all volunteers. Our job is to ensure their anonymity and keep the double blind secret. »In the airlock, Jeremy is already putting his combination. Corinne decodes:” The test doses are prepared in this sterile area, under a laminar flow hood that permanently filters the air. Before leaving, he tells how she heard in August, when she received the candidate vaccine vials. “In the first wave it was horrible. We received boxes of medicines for respiratory problems and” end of life “. There, finally, there was hope …” of production are kept secret. We just heard they’re in France.
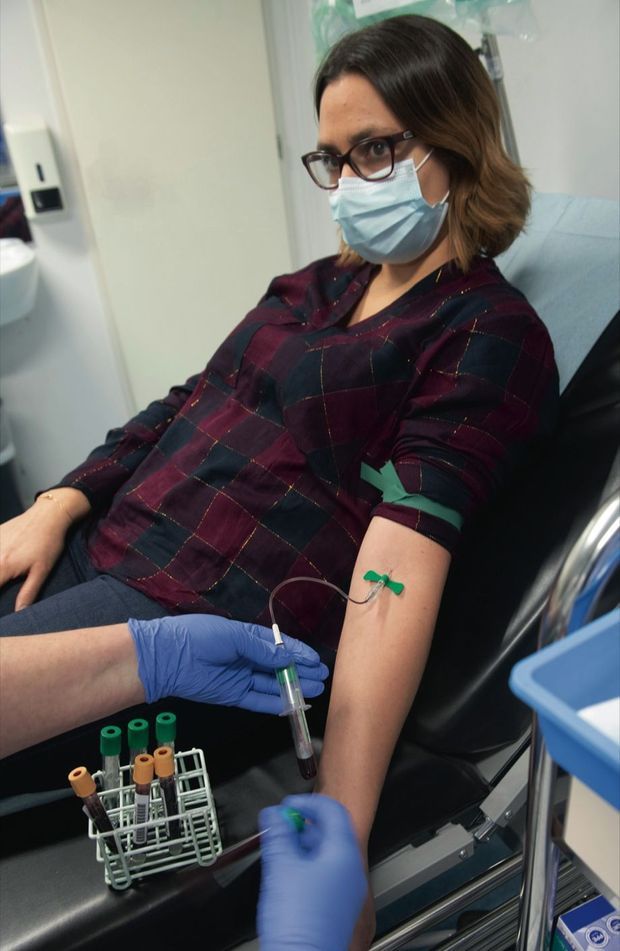
The blood test of Aurélie, 34, who has already received the two injections, will allow us to study her immune response. © Virginie Clavières / Paris Match
At the center of clinical investigations, the reception is always full. The engineer of a research laboratory, Aurélie, 34, has already received the two injections of the Phase 1 protocol. She comes for a medical examination: “Temperature OK. Perfect constants. Let’s go to the blood test, are you ready? The nurse asks. Aurélie smiles: “Last time there were 18 hits. There are only 8! “A health check-up is needed between the two injections to analyze the candidate’s immunological background. Aurélie will only know in twelve or thirteen months whether she has received the vaccine or the placebo. She doesn’t care about the side effects. His collected blood will be sent to the biological research center, then to an independent laboratory tasked with studying his immune response. “They analyze the antibodies developed to see if they are neutralizing themselves against Covid-19,” explains Odile Launay, who coordinates the protocols. But not that … They are also interested in cellular response. Lymphocytes play an important role in the response to infections. Can we hope for a vaccine in 2021? It’s possible. In principle, development takes years. But there, everything goes very quickly. This is scientifically unprecedented. In my entire career, I had never seen him. “For this vaccine, which was licensed by the US laboratory MSD (Merck Sharp and Dohme), we are moving from an academic to an industrial phase.
“One hundred and thirty-five years after the discovery of the rabies vaccine, our teams are giving their all”, vibrates Christophe d’Enfert, scientific director
At the Institut Pasteur, where it all began, the emotion is palpable. “One hundred and thirty-five years after the discovery of the rabies vaccine, our teams are doing their best,” vibrates Christophe d’Enfert, scientific director. Everyone is waiting to rest until the vaccine is on the market. We haven’t arrived yet. If the results are positive, the phase 3 trial to evaluate the protection conferred could begin in the first quarter of 2021. We will therefore need at least 30,000 participants. More than 35,000 candidates have already registered on Inserm’s Covireivac platform. 25% of them are over 65. “We will also need young people and people with chronic diseases that expose them to the risk of severe forms”, predicts Odile Launay. The tests of this last phase depend on the circulation of the virus. To assess the effectiveness of a vaccine, participants must be infected in real life, despite normal protective measures. “
How will permissions be granted? How to orchestrate the vaccination of the population? We know the Germans will commandeer hangars and vaccinate en masse. Italians are thinking of resorting to doctors and pharmacists. In France, we don’t know yet, but treating doctors could play an important role. “Can’t we go faster?” Thomas was impatient before. “More quickly may be the ability to voluntarily inoculate the virus to participants so that protection can be tested immediately,” Odile Launay replied. It has a name: “tests of human challenge”. France has positioned itself against carrying out this type of test, even though the British have given the green light to set them up. Which raises ethical questions. Can we prioritize the collective advantage, the health emergency over an individual risk? I do not know the answer. Not even Thomas. It is not known whether the liquid given to him contains the vaccine or its placebo. We look at him as a hero. He, more humbly, asks only one question: “Will he arrive in time for the meeting that awaits him?” ”
Any reproduction prohibited
Source link