[ad_1]

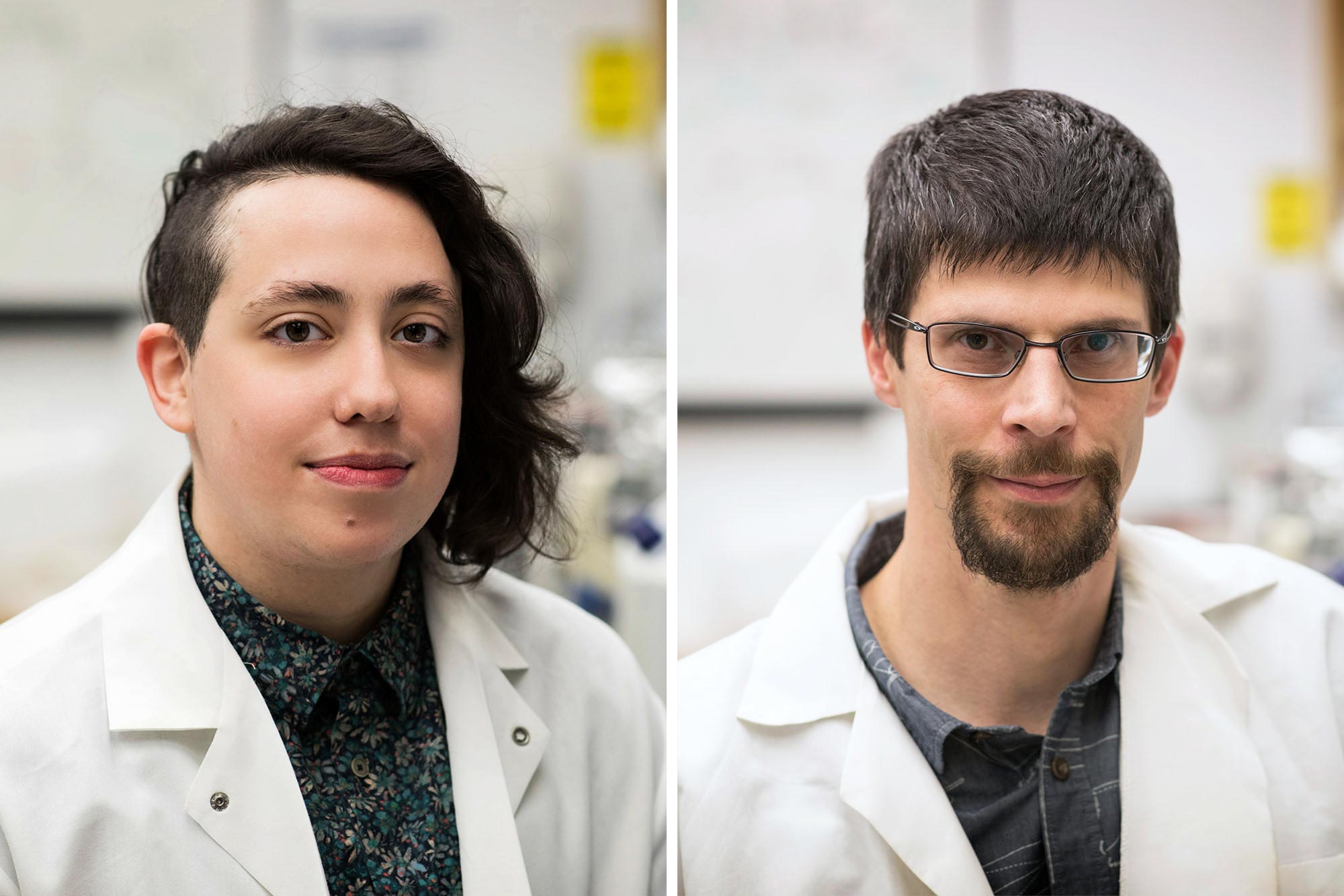
A discovery by UVA researchers Dorian A. Rosen and Alban Gaultier led to a recent clinical study that found that an antidepressant can stop COVID symptoms from worsening. Credit: photo by Dan Addison, University of Virginia Communications
The antidepressant fluvoxamine appears to prevent COVID-19 infections get worse and can help keep patients out of the hospital, suggests a study based on research from the University of Virginia School of Medicine.
The clinical trial, conducted by the Washington University School of Medicine in St. Louis, compared fluvoxamine with a placebo in 152 adult outpatients infected with the coronavirus. None of the participants who received fluvoxamine saw “clinical deterioration” after 15 days, while six patients who received placebo did. Of these six, four were hospitalized for periods ranging from four to 21 days. One was on a fan for 10 days.
Although the study size was small, the researchers say the findings are statistically significant and that fluvoxamine deserves further study as a COVID-19 treatment. They plan to launch a larger trial in the coming weeks.
“Patients who took fluvoxamine did not develop severe breathing difficulties or required hospitalization for problems with lung function,” said Eric J. Lenze, MD, of Washington University School of Medicine. “Most of the experimental treatments for COVID-19 have been aimed at the sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or have to go to hospital. Our study suggests that fluvoxamine may help fill that niche. “
Fluvoxamine and COVID-19
Washington University researchers launched the double-blind randomized study based on a discovery by Alban Gaultier, PhD, UVA and former graduate student Dorian A Rosen, PhD. Gaultier and Rosen discovered last year that fluvoxamine can stop the deadly inflammation known as sepsis, in which the immune response gets out of control. The drug, they determined, reduced the production of cytokines, which have been linked to potentially deadly “cytokine storms” believed to occur in severe cases of COVID-19.
This connection prompted the Washington University team to investigate the possibility that fluvoxamine could have a protective effect for patients with COVID-19. Perhaps, they thought, the drug could help prevent the overreactions of the immune system triggered by this strange new coronavirus. And their work suggests it might.
“Because elevated cytokine levels have been associated with COVID-19 severity, testing fluvoxamine in a clinical trial made a lot of sense to us,” said Gaultier, of the UVA Department of Neuroscience and its Center for Brain Immunology and the Glia (BIG). “We are not yet clear on how fluvoxamine works against SARS-CoV-2, but searches are underway to find the answer. “
The Washington University team noted that recent research has raised doubts that cytokines are indeed playing a major role in COVID-19 deaths. If not, the researchers say, fluvoxamine could have beneficial effects from some other mechanism not yet understood.
“There are several ways this drug could work to help COVID-19 patients, but we think it could most likely interact with the sigma-1 receptor to reduce the production of inflammatory molecules,” said Angela M Reiersen, MD of Washington. University. “Past research has shown that fluvoxamine can reduce inflammation in animal models of sepsis and could do something similar in our patients.”
The researchers pointed out that there were several limitations to their research. In addition to its small size, the study was hampered by other factors, including the fact that 20 percent of participants stopped responding to surveys during the 15-day trial. (The researchers determined that none of those participants needed hospitalization or visits to the emergency room, but they could not rule out that the participants sought treatment elsewhere, such as at emergency rooms.)
Due to these limitations, the researchers say the study results should not be treated as a measure of fluvoxamine’s effectiveness against COVID-19 but as an encouraging indicator that the drug requires further testing.
“If a larger (phase III) clinical trial confirmed the results, fluvoxamine would be a perfect treatment for newly diagnosed COVID patients,” Gaultier said. “Fluvoxamine is not an investigational drug, it is cheap and safe and could be available as a first line of defense to ease hospitals that are overwhelmed by the COVID health crisis.”
The researchers published their findings in Journal of the American Medical Association. The Washington University team consisted of Lenze, Caline Mattar, Charles F. Zorumski, Angela Stevens, Julie Schweiger, Ginger E. Nicol, J. Philip Miller, Lei Yang, Michael Yingling, Michael S. Avidan and Reiersen. A list of the authors’ disclosures is included in the document.
Reference: “Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19” by Eric J. Lenze, MD; Caline Mattar, MD; Charles F. Zorumski, MD; Angela Stevens, BA; Julie Schweiger; Ginger E. Nicol, MD; J. Philip Miller, AB; Lei Yang, MPH, MSIS; Michael Yingling, MS; Michael S. Avidan, MBBCh and Angela M. Reiersen, MD, MPE, 12 November 2020, Journal of the American Medical Association.
DOI:
The clinical trial was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the COVID-19 Early Treatment Fund. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bently Foundation, and the National Institutes of Health’s UL1TR002345 grant.
[ad_2]
Source link