[ad_1]
A study published in September in The Lancet Respiratory Medicine, signed by a team of Austrian researchers, reported the case of a patientis reached upis a severe form of COVID-19, treatedis successfully with a soluble form of recombinant human angiotensin-2 converting enzyme (denoted hrsACE2). Since then, other tests to have ConfirmIS the potential of this treatment.
Injection of soluble ACE2 resulted in rapid disappearance of the virus from the patient’s blood serum, nasal passages and lungs, as well as reduced inflammation due to high levels of cytokines. Furthermore, hrsACE2 did not prevent the production of neutralizing antibodies, which led to a significant clinical improvement in the patient.
Soluble ACE2 as a competitive receptor
The quest to develop a treatment and vaccine for COVID-19 is primarily to find viral replication inhibitors. But another strategy is also to block the cellular target of SARS-CoV-2: the angiotensin-2 converting enzyme (ACE2). Located on the surface of cells, ACE2 is an essential transmembrane protein for the coronavirus: the latter binds to it via its spike protein, after which it can penetrate target cells to replicate there.
ACE2 is expressed in several human organs, to varying degrees. It is highly expressed in the lungs (on the surface of type II alveolar epithelial cells), in the heart (on myocardial cells, coronary vascular endothelial cells and vascular smooth muscle), kidneys (on proximal tubule cells) and the small intestine (on enterocytes). Organs frequently affected in severe forms of the disease.
While membrane-bound ACE2 can mediate cellular entry of SARS-CoV-2, a genetically modified soluble form of ACE2, called hrsACE2, can limit the phenomenon by competing with the cellular receptor. In other words, the virus binds to this soluble form rather than the cellular receptor. Therefore, its action can reduce lung damage and the dysfunction of many other organs.
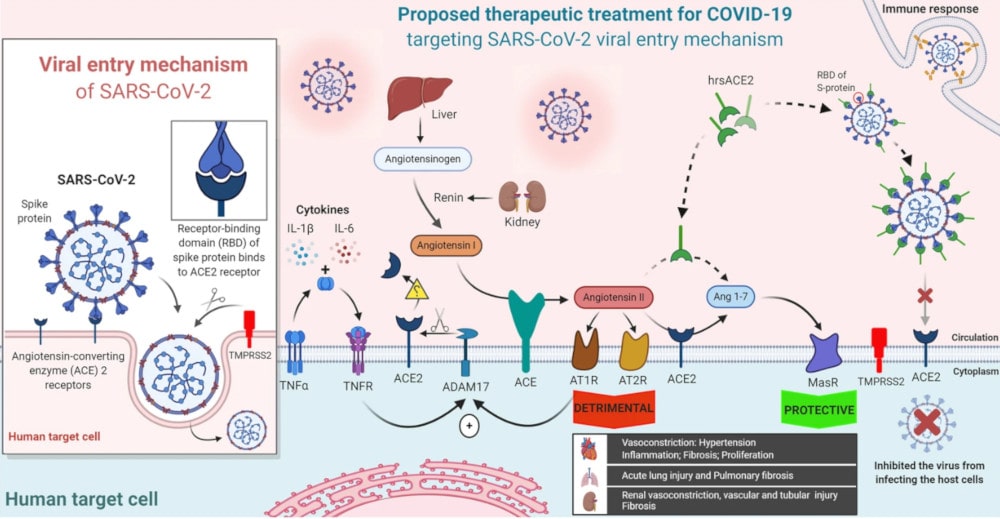
Earlier this year, you study in vitro have already shown that hrsACE2 reduced the viral growth of SARS-CoV-2 by a factor of 1000 to 5000 in cell cultures, modified human blood vessels and renal organoids. To date, hrsACE2-based treatment has been tested on 89 healthy volunteers or those with acute respiratory distress syndrome; proved effective and well tolerated. The treatment is therefore particularly promising.
The case reported in September by Dr. Alexander Zoufaly, of the Kaiser-Franz-Josef hospital in Vienna, concerns a 45-year-old woman, hospitalized with a history of cough, fatigue, muscle aches, fever and illness. severe shortness of breath for 7 days; she had also been suffering from nausea and diarrhea for 4 days. A PCR test confirmed it did indeed have COVID-19. She was first treated with hydroxychloroquine and an anticoagulant, nadroparin. But this treatment proved ineffective and did not lead to any clinical improvement in the patient’s condition.
Reduced viral load, sustained immune response
Nine days after the onset of symptoms, the patient received a dose of hrsACE2 (over 5 minutes, as an intravenous infusion), twice daily for 7 days. The product was well tolerated with no side effects. From the first dose, the researchers observed a noticeable reduction in serum angiotensin 2 levels, with concomitant increases in other types of angiotensin, including angiotensin 1–7 and angiotensin 1–9 (i.e. angiotensin each has a different biological role) . These changes lasted throughout the treatment.
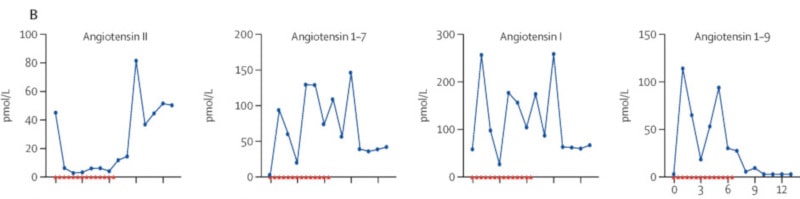
After treatment, the medical team noted a marked decrease in the concentrations of critical cytokines involved in COVID-19 (the source of the cytokine “storms”). The number of SARS-CoV-2 copies decreased considerably, from 32,000 copies per milliliter 2 days before treatment, to 2,500 and then 270 copies per milliliter after the first and second days of treatment with hrsACE2, respectively.
Furthermore, the researchers pointed out that hrsACE2 injection did not reduce the production of anti-SARS-CoV-2 IgA and IgG antibodies. Angiotensin II levels, for their part, returned to baseline (before treatment) levels within 48 hours of stopping treatment, which corresponds to previously established data on its half-life in humans. At 57 years oldis day, the patient was finally discharged from the hospital, after a significant improvement in her condition.
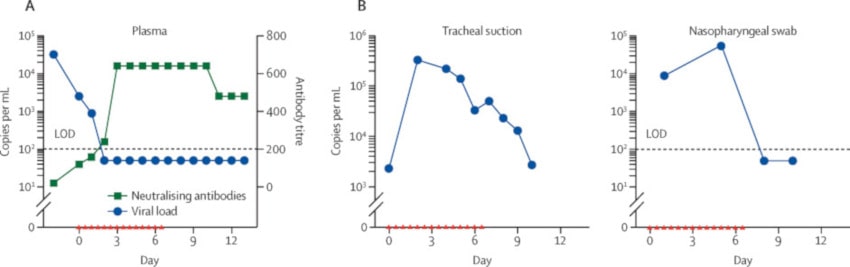
Similar data were seen in a second patient with severe COVID-19 symptoms who received two doses of hrsACE2 for 1 day. A rapid decrease in serum viral load as well as the generation of antiviral antibodies was also detected in this second patient.
Faced with these very promising clinical observations, Dr. Tarek Mohamed Abd El-Aziz, of the University of Texas Health San Antonio’s Department of Integrative and Cellular Physiology, and two collaborators believe that hrsACE2 may indeed be a solid therapeutic tool. . In addition to reducing the viral load in the respiratory system, hrsACE2 appears to play an important role in slowing or even inhibiting the spread of the virus to the lungs and other organs.
It remains to be shown that the decrease in viral load is actually due to the treatment and is not simply the result of the natural course of the disease. Additionally, although no negative side effects have been reported, El-Aziz warns that reduced angiotensin II formation, due to ACE2 overexpression, can lead to hypotension and acute kidney injury. (Angiotensin 2 plays a fundamental role in maintaining blood pressure: in particular it causes vasoconstriction and a feeling of thirst). In any case, the specialist insists that hrsACE2 deserves further research.
Source: signal transduction and targeted therapy, A. El-Aziz et al.
Source link