
[ad_1]
Artistic impression of a Coronavirus – © Marcel VFX / Pixabay
- According to a study published by our partner The Conversation, a virus must know how to adapt in order to thrive within its “host”.
- To do this, he proceeds to a modification of his cell tropism, in other words to a mutation.
- The analysis of this process was conducted by Jean-Christophe Avarre, researcher in viral ecology, and Anne-Sophie Gosselin-Grenet, professor of virology.
As we all know right now, the world’s population is recovering from a new virus causing a pandemic: SARS-CoV-2. Capable of efficient transmission, this virus quickly saturated health systems unprepared for such a threat. With its sudden appearance, this new virus is qualified as “emerging”. In virology, an emerging virus is a recently observed agent in a given population. Of animal origin, this virus has triggered an epidemic in humans, as is the case with other viruses (influenza viruses and Ebola viruses for example). This phenomenon, which allows an animal virus to become infected and multiply in humans, is linked to a mechanism called “species leap”.
What is a virus? How does it work ?
Viruses are microscopic biological entities widely distributed in the environment that play an essential role in the evolution and regulation of populations of the organisms they infect.
A virus is made up of a genome (its genetic information), a protein envelope called a capsid, which protects this genome and sometimes an envelope. A virus is unable to multiply independently, it must necessarily infect a cell to divert the raw materials and machinery it needs to manufacture its components.
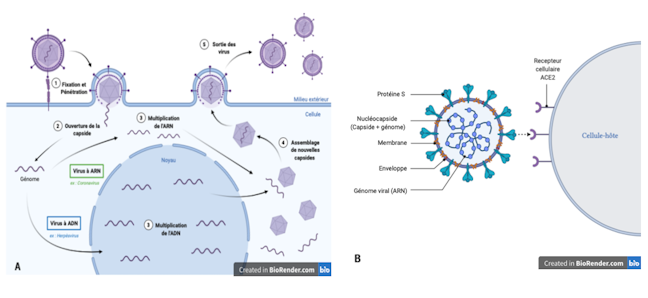
Tropism and species specificity: main viral characteristics
Infection begins with the encounter between a virus and a cell. This meeting is initiated by proteins on the surface of the virus that will recognize a specific cellular molecule, called a receptor, exposed on the cell’s surface. This recognition, which depends on the quantity and type of receptors present on the cell, defines the sensitivity of a cell towards a virus. It is essential for fixing the virus, therefore for its penetration into the cell. The multiplication of the virus will therefore depend on the permissiveness of the cell, that is, on its ability to allow the production of new viral particles.
The two parameters, sensitivity and permissiveness, thus define the cellular trophism of the virus, or its ability to penetrate and multiply preferentially in a particular type of cell.
The cells of an organism have their own unique sensitivity and permissiveness towards a virus, which also differ from species to species. The cellular trophism of the virus therefore also participates in the host spectrum of the virus, that is, in the specificity of the species it is capable of infecting and in which it can multiply. Therefore, some viruses have a broad host spectrum, while others are capable of infecting only a single host species.
Species specificity therefore implies a species barrier that prevents the passage of viruses (and pathogens in general) from one species to another and therefore the inter-species transmission of associated viral diseases. This barrier is multifactorial, at the same time physicochemical, molecular, metabolic and immunological.
Crossing a species barrier leading to viral emergence
The viral emergence can manifest itself in different ways: it can be an emergence in a new territory, linked to a change in the distribution area of the virus or its host, or the emergence of a disease in a new host species, linked to a structural modification of the virus that allows it to infect it.
A large number of viral emergencies derive from the transmission of viruses from animals to humans: we then speak of zoonotic diseases or zoonoses, as happened with AIDS resulting from the passage of viruses from monkeys to humans. Human, or for SARS of 2003, following the transmission of a bat coronavirus to humans. This inter-species transmission, or species leap, implies that the virus is able to cross the species barrier.
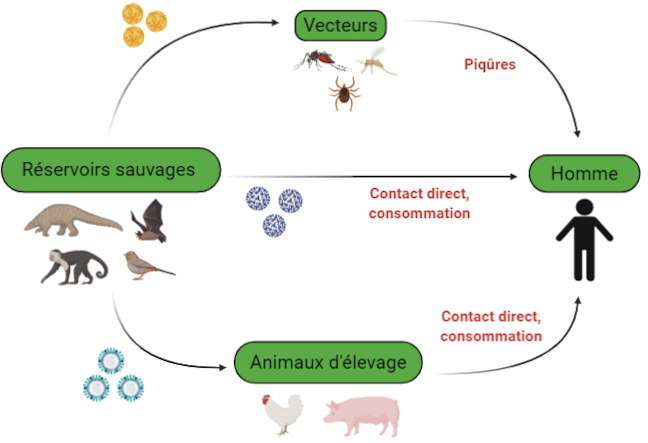 The different ways of transmission of the virus between animals and humans © DGIMI University of Montpellier
The different ways of transmission of the virus between animals and humans © DGIMI University of Montpellier
This leap of species requires close contact between a virus-infected animal species, thus qualified as a reservoir, and humans. The virus is generally not pathogenic for the reservoir and their coexistence is ancient. Viral multiplication is thus maintained in the reservoir and numerous viral particles can be produced in all harmlessness for it.
The transmission of the virus from the reservoir to humans occurs directly, in particular by ingestion of contaminated raw food or by bite, or indirectly through vectors. The latter are often arthropods, such as mosquitoes, which carry viruses between different hosts during their blood meals.
To the extent that the coexistence between the virus and the new host species is recent, the species jump may be the cause of the onset of viral diseases, as is currently the case with Covid-19.
How can species leaps occur?
In order for a species jump to be successful, the virus must perform 4 steps: to be in contact with the new host species (here humans), to infect its cells and multiply there, to escape the defenses of this host and transmit in the population of this new guest.
Contact is favored by the greater promiscuity between man and animal, which derives in particular from the expansion of metropolises, the destruction of ecosystems (deforestation) and the illegal trade in wild species.
Proximity isn’t everything, the virus has to make some changes in order to persist within the new host. Indeed, the virus must be able to adhere to the receptors on the surface of the cells of its new host to penetrate them and multiply there by diverting the cellular mechanism. This adaptability is partly allowed by their very high mutation rate. The multiplication mechanism of the viral genetic material in fact commits many errors that are not repaired by the “proofreading” systems common to living beings. These mutations can lead to structural changes in the surface proteins of the virus, allowing it to adhere to new cell types and thus altering its cellular trophism. These mutations can also make the virus able to multiply in the cells of the new host species.
The virus, exposed to the host’s defenses (its immune system), will also have to develop escape strategies. To do this, some viruses directly attack the host’s defense cells, such as HIV, others “hide” by infecting cells that are not accessible to the immune system, or even scramble the warning signs between the cells of the host organism.
Finally, to spread to the new host population, the virus must be transmitted between individuals, through respiratory droplets, blood, sexually or simply by direct contact between individuals. The strategy and efficiency of the mode of transmission will then define the ability of the virus to spread and remain in the new species. Various factors related to the infected host can also affect the efficiency of transmission. For example, once installed on the new host, the virus can use its movements to spread. Through the movements of human populations linked to globalization, trade and travel, the virus can therefore infect individuals in another region and thus extend its range. If the spread remains localized, it is called an epidemic, but if it spreads globally, then it is called a pandemic. In the event that transmission is not possible between different individuals of the species, we speak of transmission leading to an epidemiological impasse.
Through its significant impact on the environment – deforestation, poaching or intensive farming – coupled with ever-increasing globalization, humans have become a major player in the emergence of viral diseases, despite favoring normally accidental species leaps. .
This analysis was written by the students of the Master 1 Interactions Microorganismes-Hôtes-Environments at the University of Montpellier (class 2019-2020), supervised by Jean-Christophe Avarre, researcher in viral ecology at the Research Institute for Development and Anne-Sophie Gosselin-Grenet, professor of virology at the University of Montpellier.
The original article was posted on the website of The conversation.

Source link