[ad_1]
As it has been confirmed that the government has signed the first purchase agreement with AstraZeneca, a British pharmaceutical company, for a new coronavirus vaccine (Corona 19), attention is being paid to the timing of vaccination and the effectiveness of prevention in Korea. It has been confirmed that the government has signed a memorandum of understanding (MOU) with Pfizer and Johnson & Johnson.
Questions and answers on the provision of home corona vaccines
It seems it will take some time to deliver the quantity due to the overdue contract
It is not guaranteed that it is also insured with domestic consignment production
US / UK vaccination within the year Pfizer Modena
Supply negotiations in Korea are not easy
The UK government approved the emergency use of Pfizer vaccine on the 2nd, starting on the 7th as soon as possible. It is the first vaccination case in the world among phase 3 vaccines. The questions have been summarized in questions and answers.

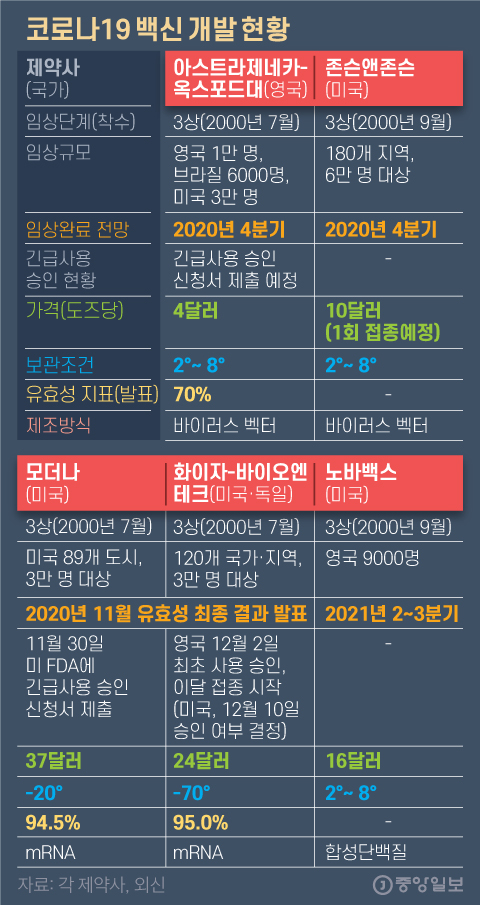
Status of Corona vaccine development 19. Graphic = Reporter Kim Young-ok [email protected]
- Is vaccination with AstraZeneca available this year?
- “No. It’s hard to play. This vaccine has not yet completed phase 3 clinical trials.”
- Can I get the vaccination early next year?
- “It doesn’t even look easy. It has to be approved for use by the Korea Food and Drug Administration and AstraZeneca has to provide the vaccine immediately, but that’s not very likely. “
Hee-seong Kim, director of the rapid review section of the Ministry of Food Safety and Medicines, said on the 3: “First of all, the final results of phase 3 have to come out. After that, if you apply for approval for the use of the vaccine at the Ministry of Food Safety and Drugs, we will review it quickly. ”If the screening goes smoothly, a permit can be issued within a month or two.
- When is AstraZeneca applied?
- “AstraZeneca has scheduled completion of Phase 3 clinical trials later this year. If you apply for vaccine approval from the KFDA in January-February next year, you can get approval for home use in March-April. “
- So can this be right?
- “Not like that. The vaccine has to arrive in Korea, but it is not known if AstraZeneca will provide it immediately.”
A pharmaceutical industry official said: “Because the supply contract is lagging behind other countries, the time for the return of the vaccine will never be soon.”
- So when can I get the vaccination?
- “Jun-wook Kwon, chief of the second vice president of central defense response headquarters, recently said:” Early in the third quarter of next year. “
- AstraZeneca vaccine is not manufactured by SK Bioscience in Korea.
- “It’s just a manufacturing contract between companies. We cannot say that we will use what was commissioned in Korea. “
- The benefits of the AstraZeneca vaccine.
- “As the storage conditions of the vaccine are 2 ~ 8 degrees, distribution and storage are easy. The storage conditions of the Pfizer vaccine are -70 degrees Celsius and Modena is -20 degrees Celsius. “
- “Compared to Pfizer and Modena, the prevention effect is lower. Pfizer and Moderna announced that the vaccine efficacy was 95% and 94.5% in the final Phase 3 clinical trial, respectively. AstraZeneca averaged 70% of the median results. However, it could further increase in the final result “.
- I have written a purchase memorandum of understanding with Pfizer and Johnson & Johnson. What is that?
- “It’s a kind of confirmation document before signing a formal contract. He made a promise to buy, but said negotiations are still ongoing regarding the purchase amount and timing of introduction. “
- The situation with Modena.
- “Modena and Pfizer are likely to be vaccinated in the US this year. However, negotiations with the Korean government are said to be not smooth. “
- Should I get the vaccine quickly?
- “National experts advise not to rush.”
In this regard, Lee Sang-won, crisis response analyst at the Korean Centers for Disease Control and Prevention, said in a briefing on the 3rd: “Vaccines take more than 10 years to be verified than to develop. . Of course, there are more safety concerns than vaccines that have been validated over a long period of time. ”
Dae-seop Song, a professor at Korea University College of Medicine, said: “The situation is different in a country with 100,000 confirmed cases per day. It is good to be fit by slowly observing safety.
Reporter Baek Minjeong and Esther [email protected]
[ad_2]
Source link